| 08-10-2016 00:19 |
Into the Night ★★★★★ ★★★★★
(21600) |
Surface Detail wrote:
jwoodward48 wrote:
Surface, do you know where I could find an emission graph for a single substance? Similar to a Planck distribution graph, but with watts measured (or similar) instead of spectral radiance. Then we could see if the distribution follows Planck or not.
IB, how about you demonstrate some of that genius scientisticalness and calculate what a 1L flask of H2 should radiate? I'm observing from 1 m away, and the flask is perfectly clear and spherical.
Because the H2 is not an opaque material, the amount of energy that your 1L flask would radiate would depend not only on the temperature and wavelength, but also on the density and pressure of the hydrogen. The more hydrogen you had in there, the more radiation you'd get. The absolute values also depend on the geometry of the apparatus; that's why line spectra never have absolute values on the y-axis. It is simply nonsensical to try to apply Planck's law to a gas. You can see that from the units of spectral radiance.
Edit: typo
Planck's law applies to the gas.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-10-2016 00:20 |
Into the Night ★★★★★ ★★★★★
(21600) |
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
IBdaMann wrote:
jwoodward48 wrote:
Yes, that's what he means.
OTTO (on the topic of) DOMAINS: if we're allowed to scale any point by emissivity, then any distribution can be turned into any other distribution! Planck is special because it's the maximum that anything can radiate - if an object has an emissivity of 1 for all wavelengths, it'll emit the Planck distribution.
What point are you trying to make?
I was just going to ask you the same question.
Have you had any luck finding a gas that doesn't radiate per Planck's, i.e it's measured E for its temperature and for a wavelength in its domain differs from what Planck's specifies?
As I've already explained, this is a nonsensical request.
Planck's law gives the spectral radiance of a surface, that is, the power output per unit of surface area per solid angle for a particular wavelength. Gas doesn't have a surface, so it doesn't have a spectral radiance. You might as well demand evidence of a solid that doesn't satisfy the ideal gas law or liquid that doesn't obey Hooke's law.
Then that means CO2 doesn't radiate!
No, it means that it doesn't radiate in accordance with Planck's Law, which applies specifically to emission from the surface of a black body. By specifying emissivity, Planck's Law can also be used for opaque surfaces that aren't perfect emitters. It cannot apply to a gas.
What's it radiating from? There is no surface!
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-10-2016 00:21 |
Into the Night ★★★★★ ★★★★★
(21600) |
jwoodward48 wrote:
Well, yes, but, Surface - oh! Line spectra! Thank you. Just the search term I needed.
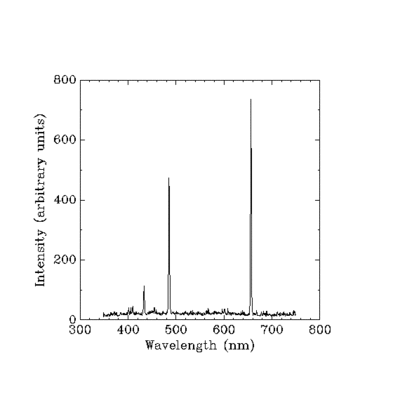
Here it is! Multiple peaks, people. You can see them with your own eyes.
Anyway, if Planck's Law still somehow applied, you could expect there to be only one peak, as this is a single substance at a single temperature. Why would you add multiple functions - there's only one! And you can see that there are multiple peaks, so... Refuted!
Nope. Looks like it follows Planck's law quite well.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-10-2016 00:22 |
Into the Night ★★★★★ ★★★★★
(21600) |
jwoodward48 wrote:
Oh, sorry, I should have specified - that's for hydrogen plasma. Also note that
...positions of lines reveal molecular properties while line strengths and shapes reveal composition, temperature, and pressure.
Also note that there are differences between the graphs of different substances. Also note that the domain of the wavelengths for this plasma includes all wavelengths from ~350 nm to ~750 nm, so cryptic DOMAIN comments don't apply.
Nothing cryptic about domains, and they do apply.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-10-2016 00:23 |
Into the Night ★★★★★ ★★★★★
(21600) |
jwoodward48 wrote:
No, I'm not asking how you take the union of two sets.
I'm asking which two domains are you combining? I can only think of one, because there's... only one radiating substance. One. Not two.
That is not how you combine domains of two different functions. You use the INTERSECTION of both sets.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
|
| 08-10-2016 00:24 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Oh, sorry, I should have specified - that's for hydrogen plasma. Also note that
...positions of lines reveal molecular properties while line strengths and shapes reveal composition, temperature, and pressure.
Also note that there are differences between the graphs of different substances. Also note that the domain of the wavelengths for this plasma includes all wavelengths from ~350 nm to ~750 nm, so cryptic DOMAIN comments don't apply.
Nothing cryptic about domains, and they do apply.
The domains aren't cryptic, your statements are.
How do they apply? The domain goes from the left to the right, no breaks or anything. Only one substance.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 08-10-2016 00:25 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
spot wrote:
IBdaMann wrote:
Surface Detail wrote: Hence black cars heat up more rapidly than white cars in sunlight, but both will cool at similar rates at night.
Do you mean the temperature of the interior of the car is affected substantially by the color of the paint on the hood and the trunk?
.
Not really controversial I think your on your own here, even ITN isn't disputing it.
Of course now he will argue in some way he is.
No, IBDaMann has got it right. The interior is affected by the color of the interior, not the hood or trunk color.
Oh, by the four hells. You're honestly claiming that car colour doesn't affect heat? How about some anecdotal evidence?
For many drivers, choosing a car color is a matter of taste, but for some car shoppers, it can be all about climate. After all, it's generally accepted that black cars are hotter in the sun and white cars keep cooler in the summer, but is it really true? Our latest video puts that theory to the test.
We took two nearly identical vehicles, one white and one black, and let them bake in the hot Georgia summer sun. When we measured the interior temperature after a few hours, we discovered this isn't just an old wives' tale. The black car's cabin measured a scorching 130 degrees Fahrenheit, while the white car's interior registered only 113 degrees.
We also decided to try answering a different question: Which one cools down faster once the air conditioning is cranked? Once again, we broke out the thermometer and discovered that the interior of the white car cooled to 84 degrees after 10 minutes, while the black car was still at 91 degrees.
Based on our test, it's safe to assume the color of your car can indeed have an impact on your comfort in the summer heat, with black cars heating up quicker -- and cooling down slower -- than white ones.
It's not scientific, but it's data. Data that lines up with everybody else's observations. You're taking on the establishment - show us your data!
The nice thing about science is that it discards anecdotal evidence.
Nothing specifies the color of the interior, which is important to the temperature of the interior.
Nothing specifies the texture of the interior, which is important to the temperature of the interior.
The color of the roof of the car can affect the interior, but not the hood or the trunk.
The air conditioning test only tests the air conditioner, not heat loss.
You have no data. I have anecdotal. You need to back your statement up with either rigorous logic and the correct application of scientific laws... or you could go and collect data better. Your choice.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 08-10-2016 00:26 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
Surface Detail wrote:
jwoodward48 wrote:
Surface, do you know where I could find an emission graph for a single substance? Similar to a Planck distribution graph, but with watts measured (or similar) instead of spectral radiance. Then we could see if the distribution follows Planck or not.
IB, how about you demonstrate some of that genius scientisticalness and calculate what a 1L flask of H2 should radiate? I'm observing from 1 m away, and the flask is perfectly clear and spherical.
Because the H2 is not an opaque material, the amount of energy that your 1L flask would radiate would depend not only on the temperature and wavelength, but also on the density and pressure of the hydrogen. The more hydrogen you had in there, the more radiation you'd get. The absolute values also depend on the geometry of the apparatus; that's why line spectra never have absolute values on the y-axis. It is simply nonsensical to try to apply Planck's law to a gas. You can see that from the units of spectral radiance.
Edit: typo
Planck's law applies to the gas.
What surface does it apply to?
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 08-10-2016 00:26 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
IBdaMann wrote:
jwoodward48 wrote:
Yes, that's what he means.
OTTO (on the topic of) DOMAINS: if we're allowed to scale any point by emissivity, then any distribution can be turned into any other distribution! Planck is special because it's the maximum that anything can radiate - if an object has an emissivity of 1 for all wavelengths, it'll emit the Planck distribution.
What point are you trying to make?
I was just going to ask you the same question.
Have you had any luck finding a gas that doesn't radiate per Planck's, i.e it's measured E for its temperature and for a wavelength in its domain differs from what Planck's specifies?
As I've already explained, this is a nonsensical request.
Planck's law gives the spectral radiance of a surface, that is, the power output per unit of surface area per solid angle for a particular wavelength. Gas doesn't have a surface, so it doesn't have a spectral radiance. You might as well demand evidence of a solid that doesn't satisfy the ideal gas law or liquid that doesn't obey Hooke's law.
Then that means CO2 doesn't radiate!
No, it means that it doesn't radiate in accordance with Planck's Law, which applies specifically to emission from the surface of a black body. By specifying emissivity, Planck's Law can also be used for opaque surfaces that aren't perfect emitters. It cannot apply to a gas.
What's it radiating from? There is no surface!
It's radiating from the middle, and the outside, and everything in between. Next?
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 08-10-2016 00:27 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Well, yes, but, Surface - oh! Line spectra! Thank you. Just the search term I needed.

Here it is! Multiple peaks, people. You can see them with your own eyes.
Anyway, if Planck's Law still somehow applied, you could expect there to be only one peak, as this is a single substance at a single temperature. Why would you add multiple functions - there's only one! And you can see that there are multiple peaks, so... Refuted!
Nope. Looks like it follows Planck's law quite well.
I... you... I am just stunned.
How does TEH MAGIK POER O' DOMAAAAAAAINS turn the Planck distribution into THAT?
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 08-10-2016 00:36 |
Surface Detail★★★★☆
(1673) |
Into the Night wrote:
Surface Detail wrote:
jwoodward48 wrote:
Surface, do you know where I could find an emission graph for a single substance? Similar to a Planck distribution graph, but with watts measured (or similar) instead of spectral radiance. Then we could see if the distribution follows Planck or not.
IB, how about you demonstrate some of that genius scientisticalness and calculate what a 1L flask of H2 should radiate? I'm observing from 1 m away, and the flask is perfectly clear and spherical.
Because the H2 is not an opaque material, the amount of energy that your 1L flask would radiate would depend not only on the temperature and wavelength, but also on the density and pressure of the hydrogen. The more hydrogen you had in there, the more radiation you'd get. The absolute values also depend on the geometry of the apparatus; that's why line spectra never have absolute values on the y-axis. It is simply nonsensical to try to apply Planck's law to a gas. You can see that from the units of spectral radiance.
Edit: typo
Planck's law applies to the gas.
How can it? According to Planck's Law, radiance depends only on temperature and wavelength. For Planck's Law to apply, the gas in the flask would have radiate with the same intensity regardless of the amount of gas in the flask, which is clearly nonsensical. |
| 08-10-2016 00:39 |
jwoodward48★★★★☆
(1537) |
It's pointless to ask that. You pretty much know what they'll say.
No, because domains, you scientifically illiterate moron. |
| 08-10-2016 00:43 |
Surface Detail★★★★☆
(1673) |
jwoodward48 wrote:
It's pointless to ask that. You pretty much know what they'll say.
No, because domains, you scientifically illiterate moron.
This weird attempt to apply set theory to radiation emission is certainly one of the odder discussions that I've had about climate change! |
| 08-10-2016 01:30 |
jwoodward48★★★★☆
(1537) |
Breaking news: IB and Into use set theory to disprove an entire branch of science. More at ten. |
| 08-10-2016 01:36 |
IBdaMann ★★★★★ ★★★★★
(14416) |
Breaking news: Creepy Clowns dupe gullible jwoodward48 into believing a WACKY religion is "settled science" as a party prank. More at ten |
|
| 08-10-2016 01:54 |
jwoodward48★★★★☆
(1537) |
At ten: They have actually disproved more than climatology - their work refutes the last century of spectroscopy. Most interesting! |
| 08-10-2016 03:12 |
Into the Night ★★★★★ ★★★★★
(21600) |
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
jwoodward48 wrote:
Surface, do you know where I could find an emission graph for a single substance? Similar to a Planck distribution graph, but with watts measured (or similar) instead of spectral radiance. Then we could see if the distribution follows Planck or not.
IB, how about you demonstrate some of that genius scientisticalness and calculate what a 1L flask of H2 should radiate? I'm observing from 1 m away, and the flask is perfectly clear and spherical.
Because the H2 is not an opaque material, the amount of energy that your 1L flask would radiate would depend not only on the temperature and wavelength, but also on the density and pressure of the hydrogen. The more hydrogen you had in there, the more radiation you'd get. The absolute values also depend on the geometry of the apparatus; that's why line spectra never have absolute values on the y-axis. It is simply nonsensical to try to apply Planck's law to a gas. You can see that from the units of spectral radiance.
Edit: typo
Planck's law applies to the gas.
How can it? According to Planck's Law, radiance depends only on temperature and wavelength. For Planck's Law to apply, the gas in the flask would have radiate with the same intensity regardless of the amount of gas in the flask, which is clearly nonsensical.
Are you saying that a flask of CO2 would radiate the same regardless of the amount of gas in the flask?
If not, why is this a significant point to bring up?
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-10-2016 03:14 |
Into the Night ★★★★★ ★★★★★
(21600) |
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Oh, sorry, I should have specified - that's for hydrogen plasma. Also note that
...positions of lines reveal molecular properties while line strengths and shapes reveal composition, temperature, and pressure.
Also note that there are differences between the graphs of different substances. Also note that the domain of the wavelengths for this plasma includes all wavelengths from ~350 nm to ~750 nm, so cryptic DOMAIN comments don't apply.
Nothing cryptic about domains, and they do apply.
The domains aren't cryptic, your statements are.
How do they apply? The domain goes from the left to the right, no breaks or anything. Only one substance.
One substance, two functions.
This has already been explained to you.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-10-2016 03:22 |
Into the Night ★★★★★ ★★★★★
(21600) |
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
spot wrote:
IBdaMann wrote:
Surface Detail wrote: Hence black cars heat up more rapidly than white cars in sunlight, but both will cool at similar rates at night.
Do you mean the temperature of the interior of the car is affected substantially by the color of the paint on the hood and the trunk?
.
Not really controversial I think your on your own here, even ITN isn't disputing it.
Of course now he will argue in some way he is.
No, IBDaMann has got it right. The interior is affected by the color of the interior, not the hood or trunk color.
Oh, by the four hells. You're honestly claiming that car colour doesn't affect heat? How about some anecdotal evidence?
For many drivers, choosing a car color is a matter of taste, but for some car shoppers, it can be all about climate. After all, it's generally accepted that black cars are hotter in the sun and white cars keep cooler in the summer, but is it really true? Our latest video puts that theory to the test.
We took two nearly identical vehicles, one white and one black, and let them bake in the hot Georgia summer sun. When we measured the interior temperature after a few hours, we discovered this isn't just an old wives' tale. The black car's cabin measured a scorching 130 degrees Fahrenheit, while the white car's interior registered only 113 degrees.
We also decided to try answering a different question: Which one cools down faster once the air conditioning is cranked? Once again, we broke out the thermometer and discovered that the interior of the white car cooled to 84 degrees after 10 minutes, while the black car was still at 91 degrees.
Based on our test, it's safe to assume the color of your car can indeed have an impact on your comfort in the summer heat, with black cars heating up quicker -- and cooling down slower -- than white ones.
It's not scientific, but it's data. Data that lines up with everybody else's observations. You're taking on the establishment - show us your data!
The nice thing about science is that it discards anecdotal evidence.
Nothing specifies the color of the interior, which is important to the temperature of the interior.
Nothing specifies the texture of the interior, which is important to the temperature of the interior.
The color of the roof of the car can affect the interior, but not the hood or the trunk.
The air conditioning test only tests the air conditioner, not heat loss.
You have no data. I have anecdotal. You need to back your statement up with either rigorous logic and the correct application of scientific laws... or you could go and collect data better. Your choice.
Actually, I no longer need data. I simply apply the theory that's already there.
But if you like anecdotal data, I happen to have two Subaru Foresters, one black and one white. They both have light gray interiors. The interior temperature between them is actually quite similar with the black one about 10 degrees warmer on an 80 degree day with full sun and no wind conditions, probably because of heat conducted via the roof of the vehicle into the interior. A slight breeze brings the temperatures of both cars to near the same. (Windows are closed)
I like Subaru cars, you see.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-10-2016 03:23 |
Into the Night ★★★★★ ★★★★★
(21600) |
jwoodward48 wrote:
Into the Night wrote:
Surface Detail wrote:
jwoodward48 wrote:
Surface, do you know where I could find an emission graph for a single substance? Similar to a Planck distribution graph, but with watts measured (or similar) instead of spectral radiance. Then we could see if the distribution follows Planck or not.
IB, how about you demonstrate some of that genius scientisticalness and calculate what a 1L flask of H2 should radiate? I'm observing from 1 m away, and the flask is perfectly clear and spherical.
Because the H2 is not an opaque material, the amount of energy that your 1L flask would radiate would depend not only on the temperature and wavelength, but also on the density and pressure of the hydrogen. The more hydrogen you had in there, the more radiation you'd get. The absolute values also depend on the geometry of the apparatus; that's why line spectra never have absolute values on the y-axis. It is simply nonsensical to try to apply Planck's law to a gas. You can see that from the units of spectral radiance.
Edit: typo
Planck's law applies to the gas.
What surface does it apply to?
Good question. The same question could be asked for CO2 radiation.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-10-2016 03:24 |
Into the Night ★★★★★ ★★★★★
(21600) |
jwoodward48 wrote:
Into the Night wrote:
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
IBdaMann wrote:
jwoodward48 wrote:
Yes, that's what he means.
OTTO (on the topic of) DOMAINS: if we're allowed to scale any point by emissivity, then any distribution can be turned into any other distribution! Planck is special because it's the maximum that anything can radiate - if an object has an emissivity of 1 for all wavelengths, it'll emit the Planck distribution.
What point are you trying to make?
I was just going to ask you the same question.
Have you had any luck finding a gas that doesn't radiate per Planck's, i.e it's measured E for its temperature and for a wavelength in its domain differs from what Planck's specifies?
As I've already explained, this is a nonsensical request.
Planck's law gives the spectral radiance of a surface, that is, the power output per unit of surface area per solid angle for a particular wavelength. Gas doesn't have a surface, so it doesn't have a spectral radiance. You might as well demand evidence of a solid that doesn't satisfy the ideal gas law or liquid that doesn't obey Hooke's law.
Then that means CO2 doesn't radiate!
No, it means that it doesn't radiate in accordance with Planck's Law, which applies specifically to emission from the surface of a black body. By specifying emissivity, Planck's Law can also be used for opaque surfaces that aren't perfect emitters. It cannot apply to a gas.
What's it radiating from? There is no surface!
It's radiating from the middle, and the outside, and everything in between. Next?
So...the gas is radiating from the effective 'surface' of every molecule in the gas. Is that right?
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-10-2016 03:26 |
Into the Night ★★★★★ ★★★★★
(21600) |
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Well, yes, but, Surface - oh! Line spectra! Thank you. Just the search term I needed.

Here it is! Multiple peaks, people. You can see them with your own eyes.
Anyway, if Planck's Law still somehow applied, you could expect there to be only one peak, as this is a single substance at a single temperature. Why would you add multiple functions - there's only one! And you can see that there are multiple peaks, so... Refuted!
Nope. Looks like it follows Planck's law quite well.
I... you... I am just stunned.
How does TEH MAGIK POER O' DOMAAAAAAAINS turn the Planck distribution into THAT?
You could go back and read IBdaMann's explanation again, or apply the Planck's curve as one domain intersecting with the spectral graph as the other domain.
Look at the heights of the spikes. They follow the Planck curve.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-10-2016 03:28 |
Into the Night ★★★★★ ★★★★★
(21600) |
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
jwoodward48 wrote:
Surface, do you know where I could find an emission graph for a single substance? Similar to a Planck distribution graph, but with watts measured (or similar) instead of spectral radiance. Then we could see if the distribution follows Planck or not.
IB, how about you demonstrate some of that genius scientisticalness and calculate what a 1L flask of H2 should radiate? I'm observing from 1 m away, and the flask is perfectly clear and spherical.
Because the H2 is not an opaque material, the amount of energy that your 1L flask would radiate would depend not only on the temperature and wavelength, but also on the density and pressure of the hydrogen. The more hydrogen you had in there, the more radiation you'd get. The absolute values also depend on the geometry of the apparatus; that's why line spectra never have absolute values on the y-axis. It is simply nonsensical to try to apply Planck's law to a gas. You can see that from the units of spectral radiance.
Edit: typo
Planck's law applies to the gas.
How can it? According to Planck's Law, radiance depends only on temperature and wavelength. For Planck's Law to apply, the gas in the flask would have radiate with the same intensity regardless of the amount of gas in the flask, which is clearly nonsensical.
You said it yourself. Surface area. Think about that.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-10-2016 05:13 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
jwoodward48 wrote:
Surface, do you know where I could find an emission graph for a single substance? Similar to a Planck distribution graph, but with watts measured (or similar) instead of spectral radiance. Then we could see if the distribution follows Planck or not.
IB, how about you demonstrate some of that genius scientisticalness and calculate what a 1L flask of H2 should radiate? I'm observing from 1 m away, and the flask is perfectly clear and spherical.
Because the H2 is not an opaque material, the amount of energy that your 1L flask would radiate would depend not only on the temperature and wavelength, but also on the density and pressure of the hydrogen. The more hydrogen you had in there, the more radiation you'd get. The absolute values also depend on the geometry of the apparatus; that's why line spectra never have absolute values on the y-axis. It is simply nonsensical to try to apply Planck's law to a gas. You can see that from the units of spectral radiance.
Edit: typo
Planck's law applies to the gas.
How can it? According to Planck's Law, radiance depends only on temperature and wavelength. For Planck's Law to apply, the gas in the flask would have radiate with the same intensity regardless of the amount of gas in the flask, which is clearly nonsensical.
Are you saying that a flask of CO2 would radiate the same regardless of the amount of gas in the flask?
If not, why is this a significant point to bring up?
No, he's saying that "radiation is independent of concentration" is what Planck would predict.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 08-10-2016 05:13 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Oh, sorry, I should have specified - that's for hydrogen plasma. Also note that
...positions of lines reveal molecular properties while line strengths and shapes reveal composition, temperature, and pressure.
Also note that there are differences between the graphs of different substances. Also note that the domain of the wavelengths for this plasma includes all wavelengths from ~350 nm to ~750 nm, so cryptic DOMAIN comments don't apply.
Nothing cryptic about domains, and they do apply.
The domains aren't cryptic, your statements are.
How do they apply? The domain goes from the left to the right, no breaks or anything. Only one substance.
One substance, two functions.
This has already been explained to you.
No, it really hasn't. Which two functions?
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 08-10-2016 05:15 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
spot wrote:
IBdaMann wrote:
Surface Detail wrote: Hence black cars heat up more rapidly than white cars in sunlight, but both will cool at similar rates at night.
Do you mean the temperature of the interior of the car is affected substantially by the color of the paint on the hood and the trunk?
.
Not really controversial I think your on your own here, even ITN isn't disputing it.
Of course now he will argue in some way he is.
No, IBDaMann has got it right. The interior is affected by the color of the interior, not the hood or trunk color.
Oh, by the four hells. You're honestly claiming that car colour doesn't affect heat? How about some anecdotal evidence?
removed because too large
The nice thing about science is that it discards anecdotal evidence.
Nothing specifies the color of the interior, which is important to the temperature of the interior.
Nothing specifies the texture of the interior, which is important to the temperature of the interior.
The color of the roof of the car can affect the interior, but not the hood or the trunk.
The air conditioning test only tests the air conditioner, not heat loss.
You have no data. I have anecdotal. You need to back your statement up with either rigorous logic and the correct application of scientific laws... or you could go and collect data better. Your choice.
Actually, I no longer need data. I simply apply the theory that's already there.
But if you like anecdotal data, I happen to have two Subaru Foresters, one black and one white. They both have light gray interiors. The interior temperature between them is actually quite similar with the black one about 10 degrees warmer on an 80 degree day with full sun and no wind conditions, probably because of heat conducted via the roof of the vehicle into the interior. A slight breeze brings the temperatures of both cars to near the same. (Windows are closed)
I like Subaru cars, you see.
Okay. Explain how "the theory" describes the outside heating up, but conduction... somehow not doing anything.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 08-10-2016 05:16 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
Surface Detail wrote:
jwoodward48 wrote:
Surface, do you know where I could find an emission graph for a single substance? Similar to a Planck distribution graph, but with watts measured (or similar) instead of spectral radiance. Then we could see if the distribution follows Planck or not.
IB, how about you demonstrate some of that genius scientisticalness and calculate what a 1L flask of H2 should radiate? I'm observing from 1 m away, and the flask is perfectly clear and spherical.
Because the H2 is not an opaque material, the amount of energy that your 1L flask would radiate would depend not only on the temperature and wavelength, but also on the density and pressure of the hydrogen. The more hydrogen you had in there, the more radiation you'd get. The absolute values also depend on the geometry of the apparatus; that's why line spectra never have absolute values on the y-axis. It is simply nonsensical to try to apply Planck's law to a gas. You can see that from the units of spectral radiance.
Edit: typo
Planck's law applies to the gas.
What surface does it apply to?
Good question. The same question could be asked for CO2 radiation.
Stop evading.
As for your point about CO2 radiation, that is taken into account. By models. Yes. Models which exist. Models which mathematically model radiation and absorption and radiation and convection, etc. They exist.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 08-10-2016 05:30 |
IBdaMann ★★★★★ ★★★★★
(14416) |
jwoodward48 wrote:As for your point about CO2 radiation, that is taken into account. By models. Yes. Models which exist. Models which mathematically model radiation and absorption and radiation and convection, etc. They exist.
Are they falsifiable models that have survived a rigorous scientific method?
.
I don't think i can [define it]. I just kind of get a feel for the phrase. - keepit
A Spaghetti strainer with the faucet running, retains water- tmiddles
Clouds don't trap heat. Clouds block cold. - Spongy Iris
Printing dollars to pay debt doesn't increase the number of dollars. - keepit
If Venus were a black body it would have a much much lower temperature than what we found there.- tmiddles
Ah the "Valid Data" myth of ITN/IBD. - tmiddles
Ceist - I couldn't agree with you more. But when money and religion are involved, and there are people who value them above all else, then the lies begin. - trafn
You are completely misunderstanding their use of the word "accumulation"! - Climate Scientist.
The Stefan-Boltzman equation doesn't come up with the correct temperature if greenhouse gases are not considered - Hank
:*sigh* Not the "raw data" crap. - Leafsdude
IB STILL hasn't explained what Planck's Law means. Just more hand waving that it applies to everything and more asserting that the greenhouse effect 'violates' it.- Ceist |
| 08-10-2016 06:12 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
IBdaMann wrote:
jwoodward48 wrote:
Yes, that's what he means.
OTTO (on the topic of) DOMAINS: if we're allowed to scale any point by emissivity, then any distribution can be turned into any other distribution! Planck is special because it's the maximum that anything can radiate - if an object has an emissivity of 1 for all wavelengths, it'll emit the Planck distribution.
What point are you trying to make?
I was just going to ask you the same question.
Have you had any luck finding a gas that doesn't radiate per Planck's, i.e it's measured E for its temperature and for a wavelength in its domain differs from what Planck's specifies?
As I've already explained, this is a nonsensical request.
Planck's law gives the spectral radiance of a surface, that is, the power output per unit of surface area per solid angle for a particular wavelength. Gas doesn't have a surface, so it doesn't have a spectral radiance. You might as well demand evidence of a solid that doesn't satisfy the ideal gas law or liquid that doesn't obey Hooke's law.
Then that means CO2 doesn't radiate!
No, it means that it doesn't radiate in accordance with Planck's Law, which applies specifically to emission from the surface of a black body. By specifying emissivity, Planck's Law can also be used for opaque surfaces that aren't perfect emitters. It cannot apply to a gas.
What's it radiating from? There is no surface!
It's radiating from the middle, and the outside, and everything in between. Next?
So...the gas is radiating from the effective 'surface' of every molecule in the gas. Is that right?
Well, yes, but you can't apply Planck's to a single molecule. "Surface" is basically meaningless at this point, you can't draw the same conclusions you could with a normal surface.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 08-10-2016 06:15 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Well, yes, but, Surface - oh! Line spectra! Thank you. Just the search term I needed.

Here it is! Multiple peaks, people. You can see them with your own eyes.
Anyway, if Planck's Law still somehow applied, you could expect there to be only one peak, as this is a single substance at a single temperature. Why would you add multiple functions - there's only one! And you can see that there are multiple peaks, so... Refuted!
Nope. Looks like it follows Planck's law quite well.
I... you... I am just stunned.
How does TEH MAGIK POER O' DOMAAAAAAAINS turn the Planck distribution into THAT?
You could go back and read IBdaMann's explanation again, or apply the Planck's curve as one domain intersecting with the spectral graph as the other domain.
Look at the heights of the spikes. They follow the Planck curve.
Care to link to that explanation? I never saw anything beyond "DOMAINS, you moron!"
What other spectral graph would there be? There's Planck's, and there's... what else?
The heights of the spikes certainly do appear Planckian, but unless you're suggesting that each spike means a molecule at a particular temperature (which isn't a thing), that comparison isn't useful. Planck only predicts one curve for a body, and that curve has one peak. At one temperature, and a single substance, Planck can't predict multiple peaks.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
|
| 08-10-2016 06:20 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
jwoodward48 wrote:
Surface, do you know where I could find an emission graph for a single substance? Similar to a Planck distribution graph, but with watts measured (or similar) instead of spectral radiance. Then we could see if the distribution follows Planck or not.
IB, how about you demonstrate some of that genius scientisticalness and calculate what a 1L flask of H2 should radiate? I'm observing from 1 m away, and the flask is perfectly clear and spherical.
Because the H2 is not an opaque material, the amount of energy that your 1L flask would radiate would depend not only on the temperature and wavelength, but also on the density and pressure of the hydrogen. The more hydrogen you had in there, the more radiation you'd get. The absolute values also depend on the geometry of the apparatus; that's why line spectra never have absolute values on the y-axis. It is simply nonsensical to try to apply Planck's law to a gas. You can see that from the units of spectral radiance.
Edit: typo
Planck's law applies to the gas.
How can it? According to Planck's Law, radiance depends only on temperature and wavelength. For Planck's Law to apply, the gas in the flask would have radiate with the same intensity regardless of the amount of gas in the flask, which is clearly nonsensical.
You said it yourself. Surface area. Think about that.
The words "surface area" don't appear in his post, and as he's already explained, gases don't have surface areas. They have effective radiating surfaces, but not every gas-body (as in a volume of a certain gas) has one of those - they need to be somewhat thick.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 08-10-2016 06:21 |
jwoodward48★★★★☆
(1537) |
IBdaMann wrote:
jwoodward48 wrote:As for your point about CO2 radiation, that is taken into account. By models. Yes. Models which exist. Models which mathematically model radiation and absorption and radiation and convection, etc. They exist.
Are they falsifiable models that have survived a rigorous scientific method?
Well, since we can measure radiation, I'd say sure, they're falsifiable. As for the "rigorous scientific method," since I didn't personally observe the scientists at work, who knows? It could all be a hoax.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 08-10-2016 17:00 |
IBdaMann ★★★★★ ★★★★★
(14416) |
jwoodward48 wrote:
IBdaMann wrote:
jwoodward48 wrote:As for your point about CO2 radiation, that is taken into account. By models. Yes. Models which exist. Models which mathematically model radiation and absorption and radiation and convection, etc. They exist.
Are they falsifiable models that have survived a rigorous scientific method?
Well, since we can measure radiation, I'd say sure, they're falsifiable. As for the "rigorous scientific method," since I didn't personally observe the scientists at work, who knows? It could all be a hoax.
It appears you have wandered into an area without any clue. Would you like some help?
.
I don't think i can [define it]. I just kind of get a feel for the phrase. - keepit
A Spaghetti strainer with the faucet running, retains water- tmiddles
Clouds don't trap heat. Clouds block cold. - Spongy Iris
Printing dollars to pay debt doesn't increase the number of dollars. - keepit
If Venus were a black body it would have a much much lower temperature than what we found there.- tmiddles
Ah the "Valid Data" myth of ITN/IBD. - tmiddles
Ceist - I couldn't agree with you more. But when money and religion are involved, and there are people who value them above all else, then the lies begin. - trafn
You are completely misunderstanding their use of the word "accumulation"! - Climate Scientist.
The Stefan-Boltzman equation doesn't come up with the correct temperature if greenhouse gases are not considered - Hank
:*sigh* Not the "raw data" crap. - Leafsdude
IB STILL hasn't explained what Planck's Law means. Just more hand waving that it applies to everything and more asserting that the greenhouse effect 'violates' it.- Ceist |
| 08-10-2016 17:27 |
jwoodward48★★★★☆
(1537) |
Sure...? |
| 08-10-2016 21:29 |
Into the Night ★★★★★ ★★★★★
(21600) |
jwoodward48 wrote:
Into the Night wrote:
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
jwoodward48 wrote:
Surface, do you know where I could find an emission graph for a single substance? Similar to a Planck distribution graph, but with watts measured (or similar) instead of spectral radiance. Then we could see if the distribution follows Planck or not.
IB, how about you demonstrate some of that genius scientisticalness and calculate what a 1L flask of H2 should radiate? I'm observing from 1 m away, and the flask is perfectly clear and spherical.
Because the H2 is not an opaque material, the amount of energy that your 1L flask would radiate would depend not only on the temperature and wavelength, but also on the density and pressure of the hydrogen. The more hydrogen you had in there, the more radiation you'd get. The absolute values also depend on the geometry of the apparatus; that's why line spectra never have absolute values on the y-axis. It is simply nonsensical to try to apply Planck's law to a gas. You can see that from the units of spectral radiance.
Edit: typo
Planck's law applies to the gas.
How can it? According to Planck's Law, radiance depends only on temperature and wavelength. For Planck's Law to apply, the gas in the flask would have radiate with the same intensity regardless of the amount of gas in the flask, which is clearly nonsensical.
Are you saying that a flask of CO2 would radiate the same regardless of the amount of gas in the flask?
If not, why is this a significant point to bring up?
No, he's saying that "radiation is independent of concentration" is what Planck would predict.
Why are you saying Planck would predict that?
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-10-2016 21:31 |
Into the Night ★★★★★ ★★★★★
(21600) |
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
spot wrote:
IBdaMann wrote:
Surface Detail wrote: Hence black cars heat up more rapidly than white cars in sunlight, but both will cool at similar rates at night.
Do you mean the temperature of the interior of the car is affected substantially by the color of the paint on the hood and the trunk?
.
Not really controversial I think your on your own here, even ITN isn't disputing it.
Of course now he will argue in some way he is.
No, IBDaMann has got it right. The interior is affected by the color of the interior, not the hood or trunk color.
Oh, by the four hells. You're honestly claiming that car colour doesn't affect heat? How about some anecdotal evidence?
removed because too large
The nice thing about science is that it discards anecdotal evidence.
Nothing specifies the color of the interior, which is important to the temperature of the interior.
Nothing specifies the texture of the interior, which is important to the temperature of the interior.
The color of the roof of the car can affect the interior, but not the hood or the trunk.
The air conditioning test only tests the air conditioner, not heat loss.
You have no data. I have anecdotal. You need to back your statement up with either rigorous logic and the correct application of scientific laws... or you could go and collect data better. Your choice.
Actually, I no longer need data. I simply apply the theory that's already there.
But if you like anecdotal data, I happen to have two Subaru Foresters, one black and one white. They both have light gray interiors. The interior temperature between them is actually quite similar with the black one about 10 degrees warmer on an 80 degree day with full sun and no wind conditions, probably because of heat conducted via the roof of the vehicle into the interior. A slight breeze brings the temperatures of both cars to near the same. (Windows are closed)
I like Subaru cars, you see.
Okay. Explain how "the theory" describes the outside heating up, but conduction... somehow not doing anything.
It is. So does convection. So does radiation. All three are in play.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-10-2016 21:34 |
Into the Night ★★★★★ ★★★★★
(21600) |
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Oh, sorry, I should have specified - that's for hydrogen plasma. Also note that
...positions of lines reveal molecular properties while line strengths and shapes reveal composition, temperature, and pressure.
Also note that there are differences between the graphs of different substances. Also note that the domain of the wavelengths for this plasma includes all wavelengths from ~350 nm to ~750 nm, so cryptic DOMAIN comments don't apply.
Nothing cryptic about domains, and they do apply.
The domains aren't cryptic, your statements are.
How do they apply? The domain goes from the left to the right, no breaks or anything. Only one substance.
One substance, two functions.
This has already been explained to you.
No, it really hasn't. Which two functions?
Already explained to you. I have repeated IBDaMann's explanation of the two functions involved, you just keep ignoring it. I'm not going to just repeat myself to satisfy your obstinance.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-10-2016 21:37 |
Into the Night ★★★★★ ★★★★★
(21600) |
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
IBdaMann wrote:
jwoodward48 wrote:
Yes, that's what he means.
OTTO (on the topic of) DOMAINS: if we're allowed to scale any point by emissivity, then any distribution can be turned into any other distribution! Planck is special because it's the maximum that anything can radiate - if an object has an emissivity of 1 for all wavelengths, it'll emit the Planck distribution.
What point are you trying to make?
I was just going to ask you the same question.
Have you had any luck finding a gas that doesn't radiate per Planck's, i.e it's measured E for its temperature and for a wavelength in its domain differs from what Planck's specifies?
As I've already explained, this is a nonsensical request.
Planck's law gives the spectral radiance of a surface, that is, the power output per unit of surface area per solid angle for a particular wavelength. Gas doesn't have a surface, so it doesn't have a spectral radiance. You might as well demand evidence of a solid that doesn't satisfy the ideal gas law or liquid that doesn't obey Hooke's law.
Then that means CO2 doesn't radiate!
No, it means that it doesn't radiate in accordance with Planck's Law, which applies specifically to emission from the surface of a black body. By specifying emissivity, Planck's Law can also be used for opaque surfaces that aren't perfect emitters. It cannot apply to a gas.
What's it radiating from? There is no surface!
It's radiating from the middle, and the outside, and everything in between. Next?
So...the gas is radiating from the effective 'surface' of every molecule in the gas. Is that right?
Well, yes, but you can't apply Planck's to a single molecule. "Surface" is basically meaningless at this point, you can't draw the same conclusions you could with a normal surface.
But a molecule has a definite size. Are you saying something with a finite size has no effective surface?
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-10-2016 21:38 |
Into the Night ★★★★★ ★★★★★
(21600) |
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Well, yes, but, Surface - oh! Line spectra! Thank you. Just the search term I needed.

Here it is! Multiple peaks, people. You can see them with your own eyes.
Anyway, if Planck's Law still somehow applied, you could expect there to be only one peak, as this is a single substance at a single temperature. Why would you add multiple functions - there's only one! And you can see that there are multiple peaks, so... Refuted!
Nope. Looks like it follows Planck's law quite well.
I... you... I am just stunned.
How does TEH MAGIK POER O' DOMAAAAAAAINS turn the Planck distribution into THAT?
You could go back and read IBdaMann's explanation again, or apply the Planck's curve as one domain intersecting with the spectral graph as the other domain.
Look at the heights of the spikes. They follow the Planck curve.
Care to link to that explanation? I never saw anything beyond "DOMAINS, you moron!"
What other spectral graph would there be? There's Planck's, and there's... what else?
The heights of the spikes certainly do appear Planckian, but unless you're suggesting that each spike means a molecule at a particular temperature (which isn't a thing), that comparison isn't useful. Planck only predicts one curve for a body, and that curve has one peak. At one temperature, and a single substance, Planck can't predict multiple peaks.
Why not?
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-10-2016 21:45 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
jwoodward48 wrote:
Surface, do you know where I could find an emission graph for a single substance? Similar to a Planck distribution graph, but with watts measured (or similar) instead of spectral radiance. Then we could see if the distribution follows Planck or not.
IB, how about you demonstrate some of that genius scientisticalness and calculate what a 1L flask of H2 should radiate? I'm observing from 1 m away, and the flask is perfectly clear and spherical.
Because the H2 is not an opaque material, the amount of energy that your 1L flask would radiate would depend not only on the temperature and wavelength, but also on the density and pressure of the hydrogen. The more hydrogen you had in there, the more radiation you'd get. The absolute values also depend on the geometry of the apparatus; that's why line spectra never have absolute values on the y-axis. It is simply nonsensical to try to apply Planck's law to a gas. You can see that from the units of spectral radiance.
Edit: typo
Planck's law applies to the gas.
How can it? According to Planck's Law, radiance depends only on temperature and wavelength. For Planck's Law to apply, the gas in the flask would have radiate with the same intensity regardless of the amount of gas in the flask, which is clearly nonsensical.
Are you saying that a flask of CO2 would radiate the same regardless of the amount of gas in the flask?
If not, why is this a significant point to bring up?
No, he's saying that "radiation is independent of concentration" is what Planck would predict.
Why are you saying Planck would predict that?
Where in Planck is there a place to input the concentration? There isn't.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
