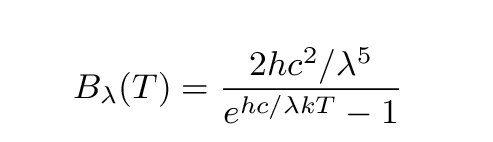| 08-10-2016 21:46 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
spot wrote:
IBdaMann wrote:
Surface Detail wrote: Hence black cars heat up more rapidly than white cars in sunlight, but both will cool at similar rates at night.
Do you mean the temperature of the interior of the car is affected substantially by the color of the paint on the hood and the trunk?
.
Not really controversial I think your on your own here, even ITN isn't disputing it.
Of course now he will argue in some way he is.
No, IBDaMann has got it right. The interior is affected by the color of the interior, not the hood or trunk color.
Oh, by the four hells. You're honestly claiming that car colour doesn't affect heat? How about some anecdotal evidence?
removed because too large
The nice thing about science is that it discards anecdotal evidence.
Nothing specifies the color of the interior, which is important to the temperature of the interior.
Nothing specifies the texture of the interior, which is important to the temperature of the interior.
The color of the roof of the car can affect the interior, but not the hood or the trunk.
The air conditioning test only tests the air conditioner, not heat loss.
You have no data. I have anecdotal. You need to back your statement up with either rigorous logic and the correct application of scientific laws... or you could go and collect data better. Your choice.
Actually, I no longer need data. I simply apply the theory that's already there.
But if you like anecdotal data, I happen to have two Subaru Foresters, one black and one white. They both have light gray interiors. The interior temperature between them is actually quite similar with the black one about 10 degrees warmer on an 80 degree day with full sun and no wind conditions, probably because of heat conducted via the roof of the vehicle into the interior. A slight breeze brings the temperatures of both cars to near the same. (Windows are closed)
I like Subaru cars, you see.
Okay. Explain how "the theory" describes the outside heating up, but conduction... somehow not doing anything.
It is. So does convection. So does radiation. All three are in play.
You're saying that the outside is warmer, but the inside is the same. Why wouldn't the energy be conducted to the inside?
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 08-10-2016 21:48 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Oh, sorry, I should have specified - that's for hydrogen plasma. Also note that
...positions of lines reveal molecular properties while line strengths and shapes reveal composition, temperature, and pressure.
Also note that there are differences between the graphs of different substances. Also note that the domain of the wavelengths for this plasma includes all wavelengths from ~350 nm to ~750 nm, so cryptic DOMAIN comments don't apply.
Nothing cryptic about domains, and they do apply.
The domains aren't cryptic, your statements are.
How do they apply? The domain goes from the left to the right, no breaks or anything. Only one substance.
One substance, two functions.
This has already been explained to you.
No, it really hasn't. Which two functions?
Already explained to you. I have repeated IBDaMann's explanation of the two functions involved, you just keep ignoring it. I'm not going to just repeat myself to satisfy your obstinance.
No, it hasn't been explained adequately. Just tell me which two functions! You can't even say that?! It takes less time then whining about it, even.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 08-10-2016 21:49 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
IBdaMann wrote:
jwoodward48 wrote:
Yes, that's what he means.
OTTO (on the topic of) DOMAINS: if we're allowed to scale any point by emissivity, then any distribution can be turned into any other distribution! Planck is special because it's the maximum that anything can radiate - if an object has an emissivity of 1 for all wavelengths, it'll emit the Planck distribution.
What point are you trying to make?
I was just going to ask you the same question.
Have you had any luck finding a gas that doesn't radiate per Planck's, i.e it's measured E for its temperature and for a wavelength in its domain differs from what Planck's specifies?
As I've already explained, this is a nonsensical request.
Planck's law gives the spectral radiance of a surface, that is, the power output per unit of surface area per solid angle for a particular wavelength. Gas doesn't have a surface, so it doesn't have a spectral radiance. You might as well demand evidence of a solid that doesn't satisfy the ideal gas law or liquid that doesn't obey Hooke's law.
Then that means CO2 doesn't radiate!
No, it means that it doesn't radiate in accordance with Planck's Law, which applies specifically to emission from the surface of a black body. By specifying emissivity, Planck's Law can also be used for opaque surfaces that aren't perfect emitters. It cannot apply to a gas.
What's it radiating from? There is no surface!
It's radiating from the middle, and the outside, and everything in between. Next?
So...the gas is radiating from the effective 'surface' of every molecule in the gas. Is that right?
Well, yes, but you can't apply Planck's to a single molecule. "Surface" is basically meaningless at this point, you can't draw the same conclusions you could with a normal surface.
But a molecule has a definite size. Are you saying something with a finite size has no effective surface?
The mass of gas has no surface. And molecules don't have temperature.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 08-10-2016 21:50 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Well, yes, but, Surface - oh! Line spectra! Thank you. Just the search term I needed.
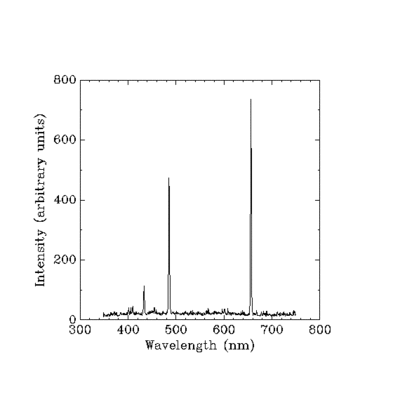
Here it is! Multiple peaks, people. You can see them with your own eyes.
Anyway, if Planck's Law still somehow applied, you could expect there to be only one peak, as this is a single substance at a single temperature. Why would you add multiple functions - there's only one! And you can see that there are multiple peaks, so... Refuted!
Nope. Looks like it follows Planck's law quite well.
I... you... I am just stunned.
How does TEH MAGIK POER O' DOMAAAAAAAINS turn the Planck distribution into THAT?
You could go back and read IBdaMann's explanation again, or apply the Planck's curve as one domain intersecting with the spectral graph as the other domain.
Look at the heights of the spikes. They follow the Planck curve.
Care to link to that explanation? I never saw anything beyond "DOMAINS, you moron!"
What other spectral graph would there be? There's Planck's, and there's... what else?
The heights of the spikes certainly do appear Planckian, but unless you're suggesting that each spike means a molecule at a particular temperature (which isn't a thing), that comparison isn't useful. Planck only predicts one curve for a body, and that curve has one peak. At one temperature, and a single substance, Planck can't predict multiple peaks.
Why not?
The Planck distribution will never have multiple peaks. It just won't. That's the way the function works.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 08-10-2016 22:07 |
Into the Night ★★★★★ ★★★★★
(21597) |
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Well, yes, but, Surface - oh! Line spectra! Thank you. Just the search term I needed.

Here it is! Multiple peaks, people. You can see them with your own eyes.
Anyway, if Planck's Law still somehow applied, you could expect there to be only one peak, as this is a single substance at a single temperature. Why would you add multiple functions - there's only one! And you can see that there are multiple peaks, so... Refuted!
Nope. Looks like it follows Planck's law quite well.
I... you... I am just stunned.
How does TEH MAGIK POER O' DOMAAAAAAAINS turn the Planck distribution into THAT?
You could go back and read IBdaMann's explanation again, or apply the Planck's curve as one domain intersecting with the spectral graph as the other domain.
Look at the heights of the spikes. They follow the Planck curve.
Care to link to that explanation? I never saw anything beyond "DOMAINS, you moron!"
What other spectral graph would there be? There's Planck's, and there's... what else?
The heights of the spikes certainly do appear Planckian, but unless you're suggesting that each spike means a molecule at a particular temperature (which isn't a thing), that comparison isn't useful. Planck only predicts one curve for a body, and that curve has one peak. At one temperature, and a single substance, Planck can't predict multiple peaks.
Why not?
The Planck distribution will never have multiple peaks. It just won't. That's the way the function works.
That's because you only considering the one function. The other is the function of the spectral lines themselves (even if you can't specify the actual function).
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
|
| 08-10-2016 22:20 |
Into the Night ★★★★★ ★★★★★
(21597) |
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
jwoodward48 wrote:
Surface, do you know where I could find an emission graph for a single substance? Similar to a Planck distribution graph, but with watts measured (or similar) instead of spectral radiance. Then we could see if the distribution follows Planck or not.
IB, how about you demonstrate some of that genius scientisticalness and calculate what a 1L flask of H2 should radiate? I'm observing from 1 m away, and the flask is perfectly clear and spherical.
Because the H2 is not an opaque material, the amount of energy that your 1L flask would radiate would depend not only on the temperature and wavelength, but also on the density and pressure of the hydrogen. The more hydrogen you had in there, the more radiation you'd get. The absolute values also depend on the geometry of the apparatus; that's why line spectra never have absolute values on the y-axis. It is simply nonsensical to try to apply Planck's law to a gas. You can see that from the units of spectral radiance.
Edit: typo
Planck's law applies to the gas.
How can it? According to Planck's Law, radiance depends only on temperature and wavelength. For Planck's Law to apply, the gas in the flask would have radiate with the same intensity regardless of the amount of gas in the flask, which is clearly nonsensical.
Are you saying that a flask of CO2 would radiate the same regardless of the amount of gas in the flask?
If not, why is this a significant point to bring up?
No, he's saying that "radiation is independent of concentration" is what Planck would predict.
Why are you saying Planck would predict that?
Where in Planck is there a place to input the concentration? There isn't.
Because you don't have to.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-10-2016 22:23 |
Into the Night ★★★★★ ★★★★★
(21597) |
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
spot wrote:
IBdaMann wrote:
Surface Detail wrote: Hence black cars heat up more rapidly than white cars in sunlight, but both will cool at similar rates at night.
Do you mean the temperature of the interior of the car is affected substantially by the color of the paint on the hood and the trunk?
.
Not really controversial I think your on your own here, even ITN isn't disputing it.
Of course now he will argue in some way he is.
No, IBDaMann has got it right. The interior is affected by the color of the interior, not the hood or trunk color.
Oh, by the four hells. You're honestly claiming that car colour doesn't affect heat? How about some anecdotal evidence?
removed because too large
The nice thing about science is that it discards anecdotal evidence.
Nothing specifies the color of the interior, which is important to the temperature of the interior.
Nothing specifies the texture of the interior, which is important to the temperature of the interior.
The color of the roof of the car can affect the interior, but not the hood or the trunk.
The air conditioning test only tests the air conditioner, not heat loss.
You have no data. I have anecdotal. You need to back your statement up with either rigorous logic and the correct application of scientific laws... or you could go and collect data better. Your choice.
Actually, I no longer need data. I simply apply the theory that's already there.
But if you like anecdotal data, I happen to have two Subaru Foresters, one black and one white. They both have light gray interiors. The interior temperature between them is actually quite similar with the black one about 10 degrees warmer on an 80 degree day with full sun and no wind conditions, probably because of heat conducted via the roof of the vehicle into the interior. A slight breeze brings the temperatures of both cars to near the same. (Windows are closed)
I like Subaru cars, you see.
Okay. Explain how "the theory" describes the outside heating up, but conduction... somehow not doing anything.
It is. So does convection. So does radiation. All three are in play.
You're saying that the outside is warmer, but the inside is the same. Why wouldn't the energy be conducted to the inside?
It is. Under no wind conditions, the black car does get a bit hotter. If there is a breeze, too much of that extra absorption is lost by conduction and convection to the moving air before it can conduct inside and the black car is basically the same as the white car.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-10-2016 22:25 |
Into the Night ★★★★★ ★★★★★
(21597) |
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
IBdaMann wrote:
jwoodward48 wrote:
Yes, that's what he means.
OTTO (on the topic of) DOMAINS: if we're allowed to scale any point by emissivity, then any distribution can be turned into any other distribution! Planck is special because it's the maximum that anything can radiate - if an object has an emissivity of 1 for all wavelengths, it'll emit the Planck distribution.
What point are you trying to make?
I was just going to ask you the same question.
Have you had any luck finding a gas that doesn't radiate per Planck's, i.e it's measured E for its temperature and for a wavelength in its domain differs from what Planck's specifies?
As I've already explained, this is a nonsensical request.
Planck's law gives the spectral radiance of a surface, that is, the power output per unit of surface area per solid angle for a particular wavelength. Gas doesn't have a surface, so it doesn't have a spectral radiance. You might as well demand evidence of a solid that doesn't satisfy the ideal gas law or liquid that doesn't obey Hooke's law.
Then that means CO2 doesn't radiate!
No, it means that it doesn't radiate in accordance with Planck's Law, which applies specifically to emission from the surface of a black body. By specifying emissivity, Planck's Law can also be used for opaque surfaces that aren't perfect emitters. It cannot apply to a gas.
What's it radiating from? There is no surface!
It's radiating from the middle, and the outside, and everything in between. Next?
So...the gas is radiating from the effective 'surface' of every molecule in the gas. Is that right?
Well, yes, but you can't apply Planck's to a single molecule. "Surface" is basically meaningless at this point, you can't draw the same conclusions you could with a normal surface.
But a molecule has a definite size. Are you saying something with a finite size has no effective surface?
The mass of gas has no surface. And molecules don't have temperature.
A single molecule vibrates. It has kinetic energy. The average kinetic energy of one molecule is the molecule itself. Molecules have a temperature.
A molecule is a definite size. To say a particle of finite size has no surface is nonsensical.
Perhaps we could reach an agreement on what a 'surface' really is.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-10-2016 22:50 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Well, yes, but, Surface - oh! Line spectra! Thank you. Just the search term I needed.

Here it is! Multiple peaks, people. You can see them with your own eyes.
Anyway, if Planck's Law still somehow applied, you could expect there to be only one peak, as this is a single substance at a single temperature. Why would you add multiple functions - there's only one! And you can see that there are multiple peaks, so... Refuted!
Nope. Looks like it follows Planck's law quite well.
I... you... I am just stunned.
How does TEH MAGIK POER O' DOMAAAAAAAINS turn the Planck distribution into THAT?
You could go back and read IBdaMann's explanation again, or apply the Planck's curve as one domain intersecting with the spectral graph as the other domain.
Look at the heights of the spikes. They follow the Planck curve.
Care to link to that explanation? I never saw anything beyond "DOMAINS, you moron!"
What other spectral graph would there be? There's Planck's, and there's... what else?
The heights of the spikes certainly do appear Planckian, but unless you're suggesting that each spike means a molecule at a particular temperature (which isn't a thing), that comparison isn't useful. Planck only predicts one curve for a body, and that curve has one peak. At one temperature, and a single substance, Planck can't predict multiple peaks.
Why not?
The Planck distribution will never have multiple peaks. It just won't. That's the way the function works.
That's because you only considering the one function. The other is the function of the spectral lines themselves (even if you can't specify the actual function).
Where are these other spectral lines coming from? Something... specific to the substance, like I've been saying?
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 08-10-2016 22:51 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
jwoodward48 wrote:
Surface, do you know where I could find an emission graph for a single substance? Similar to a Planck distribution graph, but with watts measured (or similar) instead of spectral radiance. Then we could see if the distribution follows Planck or not.
IB, how about you demonstrate some of that genius scientisticalness and calculate what a 1L flask of H2 should radiate? I'm observing from 1 m away, and the flask is perfectly clear and spherical.
Because the H2 is not an opaque material, the amount of energy that your 1L flask would radiate would depend not only on the temperature and wavelength, but also on the density and pressure of the hydrogen. The more hydrogen you had in there, the more radiation you'd get. The absolute values also depend on the geometry of the apparatus; that's why line spectra never have absolute values on the y-axis. It is simply nonsensical to try to apply Planck's law to a gas. You can see that from the units of spectral radiance.
Edit: typo
Planck's law applies to the gas.
How can it? According to Planck's Law, radiance depends only on temperature and wavelength. For Planck's Law to apply, the gas in the flask would have radiate with the same intensity regardless of the amount of gas in the flask, which is clearly nonsensical.
Are you saying that a flask of CO2 would radiate the same regardless of the amount of gas in the flask?
If not, why is this a significant point to bring up?
No, he's saying that "radiation is independent of concentration" is what Planck would predict.
Why are you saying Planck would predict that?
Where in Planck is there a place to input the concentration? There isn't.
Because you don't have to.
Why not? Planck predicts the same emission for two identical flasks full of H2 at the same temperature, except that one is at 2 atm and the other at 0.2. That's obviously not the case.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 08-10-2016 22:52 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
spot wrote:
IBdaMann wrote:
Surface Detail wrote: Hence black cars heat up more rapidly than white cars in sunlight, but both will cool at similar rates at night.
Do you mean the temperature of the interior of the car is affected substantially by the color of the paint on the hood and the trunk?
.
Not really controversial I think your on your own here, even ITN isn't disputing it.
Of course now he will argue in some way he is.
No, IBDaMann has got it right. The interior is affected by the color of the interior, not the hood or trunk color.
Oh, by the four hells. You're honestly claiming that car colour doesn't affect heat? How about some anecdotal evidence?
removed because too large
The nice thing about science is that it discards anecdotal evidence.
Nothing specifies the color of the interior, which is important to the temperature of the interior.
Nothing specifies the texture of the interior, which is important to the temperature of the interior.
The color of the roof of the car can affect the interior, but not the hood or the trunk.
The air conditioning test only tests the air conditioner, not heat loss.
You have no data. I have anecdotal. You need to back your statement up with either rigorous logic and the correct application of scientific laws... or you could go and collect data better. Your choice.
Actually, I no longer need data. I simply apply the theory that's already there.
But if you like anecdotal data, I happen to have two Subaru Foresters, one black and one white. They both have light gray interiors. The interior temperature between them is actually quite similar with the black one about 10 degrees warmer on an 80 degree day with full sun and no wind conditions, probably because of heat conducted via the roof of the vehicle into the interior. A slight breeze brings the temperatures of both cars to near the same. (Windows are closed)
I like Subaru cars, you see.
Okay. Explain how "the theory" describes the outside heating up, but conduction... somehow not doing anything.
It is. So does convection. So does radiation. All three are in play.
You're saying that the outside is warmer, but the inside is the same. Why wouldn't the energy be conducted to the inside?
It is. Under no wind conditions, the black car does get a bit hotter. If there is a breeze, too much of that extra absorption is lost by conduction and convection to the moving air before it can conduct inside and the black car is basically the same as the white car.
So you're saying that there is a negligible difference.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 08-10-2016 22:55 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
IBdaMann wrote:
jwoodward48 wrote:
Yes, that's what he means.
OTTO (on the topic of) DOMAINS: if we're allowed to scale any point by emissivity, then any distribution can be turned into any other distribution! Planck is special because it's the maximum that anything can radiate - if an object has an emissivity of 1 for all wavelengths, it'll emit the Planck distribution.
What point are you trying to make?
I was just going to ask you the same question.
Have you had any luck finding a gas that doesn't radiate per Planck's, i.e it's measured E for its temperature and for a wavelength in its domain differs from what Planck's specifies?
As I've already explained, this is a nonsensical request.
Planck's law gives the spectral radiance of a surface, that is, the power output per unit of surface area per solid angle for a particular wavelength. Gas doesn't have a surface, so it doesn't have a spectral radiance. You might as well demand evidence of a solid that doesn't satisfy the ideal gas law or liquid that doesn't obey Hooke's law.
Then that means CO2 doesn't radiate!
No, it means that it doesn't radiate in accordance with Planck's Law, which applies specifically to emission from the surface of a black body. By specifying emissivity, Planck's Law can also be used for opaque surfaces that aren't perfect emitters. It cannot apply to a gas.
What's it radiating from? There is no surface!
It's radiating from the middle, and the outside, and everything in between. Next?
So...the gas is radiating from the effective 'surface' of every molecule in the gas. Is that right?
Well, yes, but you can't apply Planck's to a single molecule. "Surface" is basically meaningless at this point, you can't draw the same conclusions you could with a normal surface.
But a molecule has a definite size. Are you saying something with a finite size has no effective surface?
The mass of gas has no surface. And molecules don't have temperature.
A single molecule vibrates. It has kinetic energy. The average kinetic energy of one molecule is the molecule itself. Molecules have a temperature.
A molecule is a definite size. To say a particle of finite size has no surface is nonsensical.
Perhaps we could reach an agreement on what a 'surface' really is.
If you have energy coming from within the substance, then S-B doesn't predict the correct emission.
For our purposes, it makes no sense to define a surface of a gas, when the radiation could come from within.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 09-10-2016 12:23 |
Into the Night ★★★★★ ★★★★★
(21597) |
jwoodward48 wrote:
Into the Night wrote:
It is. So does convection. So does radiation. All three are in play.
You're saying that the outside is warmer, but the inside is the same. Why wouldn't the energy be conducted to the inside?
I just said it is.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 09-10-2016 12:28 |
Into the Night ★★★★★ ★★★★★
(21597) |
jwoodward48 wrote:
Into the Night wrote:
Perhaps we could reach an agreement on what a 'surface' really is.
If you have energy coming from within the substance, then S-B doesn't predict the correct emission.
For our purposes, it makes no sense to define a surface of a gas, when the radiation could come from within.
What is the radiation coming from? What do you consider a 'surface'?
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 09-10-2016 16:22 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
It is. So does convection. So does radiation. All three are in play.
You're saying that the outside is warmer, but the inside is the same. Why wouldn't the energy be conducted to the inside?
I just said it is.
You said "all of them are in play," but didn't explain why being next to a warmer hood wouldn't increase the temperature.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
|
| 09-10-2016 16:25 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
Perhaps we could reach an agreement on what a 'surface' really is.
If you have energy coming from within the substance, then S-B doesn't predict the correct emission.
For our purposes, it makes no sense to define a surface of a gas, when the radiation could come from within.
What is the radiation coming from? What do you consider a 'surface'?
The radiation is coming from the outside-most part of the gas, and part of its inside. If the gas is thick enough (like the Sun), then radiation from the very middle might not make it out; a finitely thick layer, however, will be able to emit radiation directly to the outside.
A surface is "the outside part or uppermost layer of something (often used when describing its texture, form, or extent)", and for our radiative purposes, you can't consider a gas to have a surface in the same way that a solid does.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 09-10-2016 18:13 |
IBdaMann ★★★★★ ★★★★★
(14404) |
jwoodward48 wrote:The Planck distribution will never have multiple peaks. It just won't. That's the way the function works.
You are egregiously misinterpreting that graph, and you are insting on doing so.
We're going to have to revert back to assuming your math acumen is atrocious and that you shouldn't be involving yourself in such a discussion.
Go back and learn what a domain is.
Have you had any luck finding even a single example of a gas that is radiating in violation of Planck's, i.e. it's measured E at its temperature for a wavelength in its domain is not what Planck's specifies? There's a Nobel prize in it for you.
.
I don't think i can [define it]. I just kind of get a feel for the phrase. - keepit
A Spaghetti strainer with the faucet running, retains water- tmiddles
Clouds don't trap heat. Clouds block cold. - Spongy Iris
Printing dollars to pay debt doesn't increase the number of dollars. - keepit
If Venus were a black body it would have a much much lower temperature than what we found there.- tmiddles
Ah the "Valid Data" myth of ITN/IBD. - tmiddles
Ceist - I couldn't agree with you more. But when money and religion are involved, and there are people who value them above all else, then the lies begin. - trafn
You are completely misunderstanding their use of the word "accumulation"! - Climate Scientist.
The Stefan-Boltzman equation doesn't come up with the correct temperature if greenhouse gases are not considered - Hank
:*sigh* Not the "raw data" crap. - Leafsdude
IB STILL hasn't explained what Planck's Law means. Just more hand waving that it applies to everything and more asserting that the greenhouse effect 'violates' it.- Ceist |
| 09-10-2016 22:14 |
Surface Detail★★★★☆
(1673) |
jwoodward48 wrote:
Well, yes, but, Surface - oh! Line spectra! Thank you. Just the search term I needed.

Here it is! Multiple peaks, people. You can see them with your own eyes.
Anyway, if Planck's Law still somehow applied, you could expect there to be only one peak, as this is a single substance at a single temperature. Why would you add multiple functions - there's only one! And you can see that there are multiple peaks, so... Refuted!
The strong peak that you see in the hydrogen spectrum has a wavelength of 656.3 nm. It is referred to as the H-alpha line, and is the result of emission from hydrogen atoms reducing their energy state from the n=3 quantum level to the n=2 quantum level.
This wavelength (lambda) is calculated from the Bohr model of the atom as follows:
1/lambda = RH ( 1/n1^2 - 1/n2^2)
where RH is the Rydberg constant ( = 1.097 x 10^7 m^-1), and n1 and n2 are the final and initial quantum states.
In our case, n1 = 2 and n2 = 3, so we have
1/lambda = 1.097 x 10^7 (1/4 - 1/9) = 1.524 x 10^6 m^-1
So lambda = 1/1.524 x 10^6 m^-1 = 656.3 nm
Note that the Rydberg constant can be expressed in terms of fundamental constants as follows:
RH = 2 pi^2 m e^4 / h^2
where m = mass of electron, e = electron charge and h = Planck's constant.
So quantum physics does a pretty good job of predicting the wavelengths of hydrogen emission. It would be interesting to hear how IBdaMann and ITN are able to predict this emission using Planck's law and domains. |
| 09-10-2016 23:02 |
jwoodward48★★★★☆
(1537) |
IBdaMann wrote:
jwoodward48 wrote:The Planck distribution will never have multiple peaks. It just won't. That's the way the function works.
You are egregiously misinterpreting that graph, and you are insting on doing so.
I believe you meant "insisting," not "insting." I am neither a bee nor a wasp.
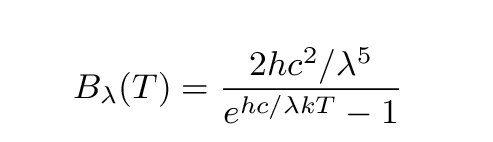
In case you couldn't tell, that's the Planck function. It will never have two peaks.

See?
We're going to have to revert back to assuming your math acumen is atrocious and that you shouldn't be involving yourself in such a discussion.
Quit the ad hominems, dude, they don't work.
Go back and learn what a domain is.
Go back and learn what a hypocrite is. Also study the Dunning-Kruger effect. It pertains to you. Yes, very much so.
Have you had any luck finding even a single example of a gas that is radiating in violation of Planck's, i.e. it's measured E at its temperature for a wavelength in its domain is not what Planck's specifies? There's a Nobel prize in it for you.
Have you had any luck calculating what the specified E is for a 1L spherical, clear flask full of H2 at 2 atm at 100K is at the 700 nm wavelength? It's being observed from 1 meter away.
Unless you can specify that, your statements are unfalsifiable. Give me something to test, and I'll test it. Don't give me anything to test, and I can't.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 09-10-2016 23:09 |
Into the Night ★★★★★ ★★★★★
(21597) |
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
It is. So does convection. So does radiation. All three are in play.
You're saying that the outside is warmer, but the inside is the same. Why wouldn't the energy be conducted to the inside?
I just said it is.
You said "all of them are in play," but didn't explain why being next to a warmer hood wouldn't increase the temperature.
Yes, I did. I described in detail how that can happen.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 09-10-2016 23:11 |
Into the Night ★★★★★ ★★★★★
(21597) |
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
Perhaps we could reach an agreement on what a 'surface' really is.
If you have energy coming from within the substance, then S-B doesn't predict the correct emission.
For our purposes, it makes no sense to define a surface of a gas, when the radiation could come from within.
What is the radiation coming from? What do you consider a 'surface'?
The radiation is coming from the outside-most part of the gas, and part of its inside. If the gas is thick enough (like the Sun), then radiation from the very middle might not make it out; a finitely thick layer, however, will be able to emit radiation directly to the outside.
A surface is "the outside part or uppermost layer of something (often used when describing its texture, form, or extent)", and for our radiative purposes, you can't consider a gas to have a surface in the same way that a solid does.
You seem to have a very vague idea of what a 'surface' is. I think you're guessing again.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 09-10-2016 23:13 |
Into the Night ★★★★★ ★★★★★
(21597) |
jwoodward48 wrote:
IBdaMann wrote:
jwoodward48 wrote:The Planck distribution will never have multiple peaks. It just won't. That's the way the function works.
You are egregiously misinterpreting that graph, and you are insting on doing so.
I believe you meant "insisting," not "insting." I am neither a bee nor a wasp.
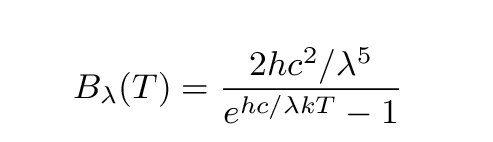
In case you couldn't tell, that's the Planck function. It will never have two peaks.

See?
We're going to have to revert back to assuming your math acumen is atrocious and that you shouldn't be involving yourself in such a discussion.
Quit the ad hominems, dude, they don't work.
Go back and learn what a domain is.
Go back and learn what a hypocrite is. Also study the Dunning-Kruger effect. It pertains to you. Yes, very much so.
Have you had any luck finding even a single example of a gas that is radiating in violation of Planck's, i.e. it's measured E at its temperature for a wavelength in its domain is not what Planck's specifies? There's a Nobel prize in it for you.
Have you had any luck calculating what the specified E is for a 1L spherical, clear flask full of H2 at 2 atm at 100K is at the 700 nm wavelength? It's being observed from 1 meter away.
Unless you can specify that, your statements are unfalsifiable. Give me something to test, and I'll test it. Don't give me anything to test, and I can't.
Again you fixating on only one function of the two involved.
Go learn about domains, dude.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 09-10-2016 23:27 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
It is. So does convection. So does radiation. All three are in play.
You're saying that the outside is warmer, but the inside is the same. Why wouldn't the energy be conducted to the inside?
I just said it is.
You said "all of them are in play," but didn't explain why being next to a warmer hood wouldn't increase the temperature.
Yes, I did. I described in detail how that can happen.
A slight breeze brings the temperatures of both cars to near the same. (Windows are closed)
Wow, look at the detail. It even explains why, if taken to the extreme, this statement would imply that everything within a breeze is at the same temperature. What an amazing orator.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 09-10-2016 23:28 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
jwoodward48 wrote:
Into the Night wrote:
Perhaps we could reach an agreement on what a 'surface' really is.
If you have energy coming from within the substance, then S-B doesn't predict the correct emission.
For our purposes, it makes no sense to define a surface of a gas, when the radiation could come from within.
What is the radiation coming from? What do you consider a 'surface'?
The radiation is coming from the outside-most part of the gas, and part of its inside. If the gas is thick enough (like the Sun), then radiation from the very middle might not make it out; a finitely thick layer, however, will be able to emit radiation directly to the outside.
A surface is "the outside part or uppermost layer of something (often used when describing its texture, form, or extent)", and for our radiative purposes, you can't consider a gas to have a surface in the same way that a solid does.
You seem to have a very vague idea of what a 'surface' is. I think you're guessing again.
Hey, I just Googled it. Notice the quotes? If you want someone to Google the definitions of basic words like "surface", I'm your guy.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 09-10-2016 23:29 |
jwoodward48★★★★☆
(1537) |
Into the Night wrote:
jwoodward48 wrote:
IBdaMann wrote:
jwoodward48 wrote:The Planck distribution will never have multiple peaks. It just won't. That's the way the function works.
You are egregiously misinterpreting that graph, and you are insting on doing so.
I believe you meant "insisting," not "insting." I am neither a bee nor a wasp.
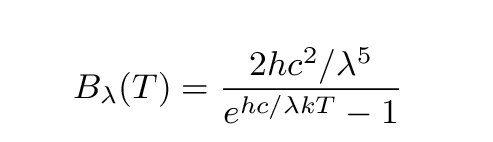
In case you couldn't tell, that's the Planck function. It will never have two peaks.

See?
We're going to have to revert back to assuming your math acumen is atrocious and that you shouldn't be involving yourself in such a discussion.
Quit the ad hominems, dude, they don't work.
Go back and learn what a domain is.
Go back and learn what a hypocrite is. Also study the Dunning-Kruger effect. It pertains to you. Yes, very much so.
Have you had any luck finding even a single example of a gas that is radiating in violation of Planck's, i.e. it's measured E at its temperature for a wavelength in its domain is not what Planck's specifies? There's a Nobel prize in it for you.
Have you had any luck calculating what the specified E is for a 1L spherical, clear flask full of H2 at 2 atm at 100K is at the 700 nm wavelength? It's being observed from 1 meter away.
Unless you can specify that, your statements are unfalsifiable. Give me something to test, and I'll test it. Don't give me anything to test, and I can't.
Again you fixating on only one function of the two involved.
Go learn about domains, dude.
What other function? You've said something about "the spectral density". That's a description of the radiation. Start using your brain, you dolt.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 10-10-2016 08:32 |
IBdaMann ★★★★★ ★★★★★
(14404) |
jwoodward48 wrote:In case you couldn't tell, that's the Planck function. It will never have two peaks.
Since you can't tell, that function will only have one local maximum if the wavelength domain contains all values.
Alter the wavelength domain, e.g. gases, and you can have many local maxima.
You might want to learn what a domain is.
As I wrote earlier, we're going to have to revert back to assuming your math acumen is atrocious and that you shouldn't be involving yourself in such a discussion, at least not yet.
jwoodward48 wrote:
Have you had any luck finding even a single example of a gas that is radiating in violation of Planck's, i.e. it's measured E at its temperature for a wavelength in its domain is not what Planck's specifies? There's a Nobel prize in it for you.
Have you had any luck calculating what the specified E is for a 1L spherical, clear flask full of H2 at 2 atm at 100K is at the 700 nm wavelength? It's being observed from 1 meter away.
So that's your long-winded way of saying that you still haven't found a single example without openly admitting that you still know of no such example.
.
I don't think i can [define it]. I just kind of get a feel for the phrase. - keepit
A Spaghetti strainer with the faucet running, retains water- tmiddles
Clouds don't trap heat. Clouds block cold. - Spongy Iris
Printing dollars to pay debt doesn't increase the number of dollars. - keepit
If Venus were a black body it would have a much much lower temperature than what we found there.- tmiddles
Ah the "Valid Data" myth of ITN/IBD. - tmiddles
Ceist - I couldn't agree with you more. But when money and religion are involved, and there are people who value them above all else, then the lies begin. - trafn
You are completely misunderstanding their use of the word "accumulation"! - Climate Scientist.
The Stefan-Boltzman equation doesn't come up with the correct temperature if greenhouse gases are not considered - Hank
:*sigh* Not the "raw data" crap. - Leafsdude
IB STILL hasn't explained what Planck's Law means. Just more hand waving that it applies to everything and more asserting that the greenhouse effect 'violates' it.- Ceist |
| 10-10-2016 10:45 |
Surface Detail★★★★☆
(1673) |
IBdaMann, all you have done is blather and insult. You clearly don't have the faintest idea what you're talking about. I have explained how, for example, basic quantum mechanics predicts the hydrogen emission peak at 656.3 nm. You have explained nothing and you have learned nothing. Science requires the ability to grasp abstract concepts. You obviously do not possess this ability. Why are you wasting your time here? |
| 10-10-2016 14:18 |
jwoodward48★★★★☆
(1537) |
IBdaMann wrote:
jwoodward48 wrote:In case you couldn't tell, that's the Planck function. It will never have two peaks.
Since you can't tell, that function will only have one local maximum if the wavelength domain contains all values.
Not true. Removing numbers from the domain will not create a new peak.
Alter the wavelength domain, e.g. gases, and you can have many local maxima.
You cannot.
You might want to learn what a domain is.
You might want to learn geometry.
As I wrote earlier, we're going to have to revert back to assuming your math acumen is atrocious and that you shouldn't be involving yourself in such a discussion, at least not yet.
As I wrote earlier, we're going to have to revert back to assuming your math acumen is atrocious and that you shouldn't be involving yourself in such a discussion, at least not yet.
jwoodward48 wrote:
Have you had any luck finding even a single example of a gas that is radiating in violation of Planck's, i.e. it's measured E at its temperature for a wavelength in its domain is not what Planck's specifies? There's a Nobel prize in it for you.
Have you had any luck calculating what the specified E is for a 1L spherical, clear flask full of H2 at 2 atm at 100K is at the 700 nm wavelength? It's being observed from 1 meter away.
So that's your long-winded way of saying that you still haven't found a single example without openly admitting that you still know of no such example.
No, it's my detailed description of an experiment. Go on, do it. Calculate. Or are your claims unfalsifiable?
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 10-10-2016 17:27 |
IBdaMann ★★★★★ ★★★★★
(14404) |
jwoodward48 wrote:Not true. Removing numbers from the domain will not create a new peak.
Yes it will. If you remove from the domain the existing peak you will necessarily be left with two new peaks on either side.
The person you trust who you are regurgitating is a moron. Is it Surface Detail?
jwoodward48 wrote:
Alter the wavelength domain, e.g. gases, and you can have many local maxima.
You cannot.
Yes you can and you are a moron. I don't know why you insist on making assertions about math you don't understand, ... but it's funny.
jwoodward48 wrote:You might want to learn geometry.
I love geometry. Let me know if you have any questions.
jwoodward48 wrote: Go on, do it. Calculate. Or are your claims unfalsifiable?
I'm not making any claims. If you're happy with "greenhouse effect" remaining an unsupported violation of physics then I'm happy as well.
.
I don't think i can [define it]. I just kind of get a feel for the phrase. - keepit
A Spaghetti strainer with the faucet running, retains water- tmiddles
Clouds don't trap heat. Clouds block cold. - Spongy Iris
Printing dollars to pay debt doesn't increase the number of dollars. - keepit
If Venus were a black body it would have a much much lower temperature than what we found there.- tmiddles
Ah the "Valid Data" myth of ITN/IBD. - tmiddles
Ceist - I couldn't agree with you more. But when money and religion are involved, and there are people who value them above all else, then the lies begin. - trafn
You are completely misunderstanding their use of the word "accumulation"! - Climate Scientist.
The Stefan-Boltzman equation doesn't come up with the correct temperature if greenhouse gases are not considered - Hank
:*sigh* Not the "raw data" crap. - Leafsdude
IB STILL hasn't explained what Planck's Law means. Just more hand waving that it applies to everything and more asserting that the greenhouse effect 'violates' it.- Ceist |
| 10-10-2016 18:00 |
Surface Detail★★★★☆
(1673) |
IBdaMann: Explain in terms of domains why hot hydrogen gas emits strongly at 656.3 nm, or admit you're taking crap and shut up. |
|
| 10-10-2016 18:05 |
jwoodward48★★★★☆
(1537) |
IB, when the domain of the measured radiation goes smoothly from one value to another, and within that interval there are multiple local maxima, you can't explain that with "a removed part of the domain" - it's intact! Nothing is missing!
And besides,
X
X X
X X
X X
This is the Planck function.
X X
X X
X X
This is the Planck function minus the peak.
X X
X X X X
This is the observed hydrogen. See? Continuous domain, multiple local maxima. |
| 10-10-2016 19:35 |
IBdaMann ★★★★★ ★★★★★
(14404) |
Surface Detail wrote:
IBdaMann: Explain in terms of domains why hot hydrogen gas emits strongly at 656.3 nm, or admit you're taking crap and shut up.
What values do you get when you run it through Planck's?
Post your work here and I'll tell you where you have errors, if any.
.
I don't think i can [define it]. I just kind of get a feel for the phrase. - keepit
A Spaghetti strainer with the faucet running, retains water- tmiddles
Clouds don't trap heat. Clouds block cold. - Spongy Iris
Printing dollars to pay debt doesn't increase the number of dollars. - keepit
If Venus were a black body it would have a much much lower temperature than what we found there.- tmiddles
Ah the "Valid Data" myth of ITN/IBD. - tmiddles
Ceist - I couldn't agree with you more. But when money and religion are involved, and there are people who value them above all else, then the lies begin. - trafn
You are completely misunderstanding their use of the word "accumulation"! - Climate Scientist.
The Stefan-Boltzman equation doesn't come up with the correct temperature if greenhouse gases are not considered - Hank
:*sigh* Not the "raw data" crap. - Leafsdude
IB STILL hasn't explained what Planck's Law means. Just more hand waving that it applies to everything and more asserting that the greenhouse effect 'violates' it.- Ceist |
| 10-10-2016 19:40 |
IBdaMann ★★★★★ ★★★★★
(14404) |
jwoodward48 wrote:
IB, when the domain of the measured radiation goes smoothly from one value to another, and within that interval there are multiple local maxima, you can't explain that with "a removed part of the domain" - it's intact! Nothing is missing!
And besides,
X
X X
X X
X X
This is the Planck function.
X X
X X
X X
This is the Planck function minus the peak.
X X
X X X X
This is the observed hydrogen. See? Continuous domain, multiple local maxima.
None of what you wrote here applies to the point in question. What a tangent.
Did you want help?
.
I don't think i can [define it]. I just kind of get a feel for the phrase. - keepit
A Spaghetti strainer with the faucet running, retains water- tmiddles
Clouds don't trap heat. Clouds block cold. - Spongy Iris
Printing dollars to pay debt doesn't increase the number of dollars. - keepit
If Venus were a black body it would have a much much lower temperature than what we found there.- tmiddles
Ah the "Valid Data" myth of ITN/IBD. - tmiddles
Ceist - I couldn't agree with you more. But when money and religion are involved, and there are people who value them above all else, then the lies begin. - trafn
You are completely misunderstanding their use of the word "accumulation"! - Climate Scientist.
The Stefan-Boltzman equation doesn't come up with the correct temperature if greenhouse gases are not considered - Hank
:*sigh* Not the "raw data" crap. - Leafsdude
IB STILL hasn't explained what Planck's Law means. Just more hand waving that it applies to everything and more asserting that the greenhouse effect 'violates' it.- Ceist |
| 10-10-2016 19:48 |
spot★★★★☆
(1323) |
IBdaMann wrote:
jwoodward48 wrote:
IB, when the domain of the measured radiation goes smoothly from one value to another, and within that interval there are multiple local maxima, you can't explain that with "a removed part of the domain" - it's intact! Nothing is missing!
And besides,
X
X X
X X
X X
This is the Planck function.
X X
X X
X X
This is the Planck function minus the peak.
X X
X X X X
This is the observed hydrogen. See? Continuous domain, multiple local maxima.
None of what you wrote here applies to the point in question. What a tangent.
Did you want help?
.
Help to understand wtf you are on about. |
| 10-10-2016 19:59 |
Surface Detail★★★★☆
(1673) |
IBdaMann wrote:
Surface Detail wrote:
IBdaMann: Explain in terms of domains why hot hydrogen gas emits strongly at 656.3 nm, or admit you're taking crap and shut up.
What values do you get when you run it through Planck's?
Post your work here and I'll tell you where you have errors, if any.
It is you, not I, who is claiming that Planck's law (and domains) determines the emission of radiation from gases. So it is you who needs to show how Planck's law (and domains) predicts the experimentally observed emission at 656.3 nm. Obviously you can't, because you're talking complete bollocks: Planck's law applies to black bodies, not gases.
I've already shown how the Bohr model of the hydrogen atom predicts this emission using basic quantum mechanics. |
| 10-10-2016 21:16 |
jwoodward48★★★★☆
(1537) |
IBdaMann wrote:
jwoodward48 wrote:
IB, when the domain of the measured radiation goes smoothly from one value to another, and within that interval there are multiple local maxima, you can't explain that with "a removed part of the domain" - it's intact! Nothing is missing!
And besides,
X
X X
X X
X X
This is the Planck function.
X X
X X
X X
This is the Planck function minus the peak.
X X
X X X X
This is the observed hydrogen. See? Continuous domain, multiple local maxima.
None of what you wrote here applies to the point in question. What a tangent.
Did you want help?
.
Oops, something messed up the format.
___X
__X_X
_X___X
X_____X
This is the Planck function.
__X_X
_X___X
X_____X
This is the Planck function minus the peak.
_X___X
X_X_X__X
This is the observed hydrogen. See? Continuous domain, multiple local maxima.
This applies to your "domain" point. There is only one domain, and it is continuous. Your point is good, but it doesn't apply here.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 10-10-2016 21:21 |
jwoodward48★★★★☆
(1537) |
IBdaMann wrote:
jwoodward48 wrote:Not true. Removing numbers from the domain will not create a new peak.
Yes it will. If you remove from the domain the existing peak you will necessarily be left with two new peaks on either side.
The person you trust who you are regurgitating is a moron. Is it Surface Detail?
Usual insults.
jwoodward48 wrote:
Alter the wavelength domain, e.g. gases, and you can have many local maxima.
You cannot.
Yes you can and you are a moron. I don't know why you insist on making assertions about math you don't understand, ... but it's funny.
You cannot produce the observed graphs, all of which have continuous domains, by removing portions of the domain of the Planck distribution.
And besides, your method will always produce at most two peaks, at the same height. That's not what we see.
jwoodward48 wrote:You might want to learn geometry.
I love geometry. Let me know if you have any questions.
Nope. I don't think there's much I can learn from you on this topic.
jwoodward48 wrote: Go on, do it. Calculate. Or are your claims unfalsifiable?
I'm not making any claims. If you're happy with "greenhouse effect" remaining an unsupported violation of physics then I'm happy as well.
By the bloody beard of Armok, you are making a claim.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 10-10-2016 21:22 |
jwoodward48★★★★☆
(1537) |
Surface Detail wrote:
IBdaMann wrote:
Surface Detail wrote:
IBdaMann: Explain in terms of domains why hot hydrogen gas emits strongly at 656.3 nm, or admit you're taking crap and shut up.
What values do you get when you run it through Planck's?
Post your work here and I'll tell you where you have errors, if any.
It is you, not I, who is claiming that Planck's law (and domains) determines the emission of radiation from gases. So it is you who needs to show how Planck's law (and domains) predicts the experimentally observed emission at 656.3 nm. Obviously you can't, because you're talking complete bollocks: Planck's law applies to black bodies, not gases.
I've already shown how the Bohr model of the hydrogen atom predicts this emission using basic quantum mechanics.
No, because domains.
"Heads on a science
Apart" - Coldplay, The Scientist
IBdaMann wrote:
No, science doesn't insist that, ergo I don't insist that.
I am the Ninja Scientist! Beware! |
| 11-10-2016 02:33 |
Into the Night ★★★★★ ★★★★★
(21597) |
Surface Detail wrote:
IBdaMann wrote:
Surface Detail wrote:
IBdaMann: Explain in terms of domains why hot hydrogen gas emits strongly at 656.3 nm, or admit you're taking crap and shut up.
What values do you get when you run it through Planck's?
Post your work here and I'll tell you where you have errors, if any.
It is you, not I, who is claiming that Planck's law (and domains) determines the emission of radiation from gases. So it is you who needs to show how Planck's law (and domains) predicts the experimentally observed emission at 656.3 nm. Obviously you can't, because you're talking complete bollocks: Planck's law applies to black bodies, not gases.
I've already shown how the Bohr model of the hydrogen atom predicts this emission using basic quantum mechanics.
Planck's law applies to gases too. You have yet to produce a gas it doesn't apply to.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 11-10-2016 03:07 |
jwoodward48★★★★☆
(1537) |
You have yet to demonstrate a calculation that could be used to falsify your claims. Would you care to take up IB's challenge? |