Greenhouse Effect is Real
| Greenhouse Effect is Real06-09-2017 07:14 | |
| GreenMan★★★☆☆ (661) |
After much debate, we finally got Into the Night, aka Parrot Killer [the infamous and notorious self proclaimed slayer of Church of Global Warming Parrots] to admit that CO2, does absorb some radiation and warm up. Here are his exact words:Into the Night Said: Into the Night Said: He is a little mistaken about "all substances do," though, since oxygen and nitrogen, which are the most abundant substances in the air do not absorb any light, which is what separates them from the Greenhouse Gases, which cause the Greenhouse Effect. So let's have a thread on the Greenhouse Effect, since it has been accepted as FACT that CO2 does indeed absorb infrared radiation from the surface of the earth and warm up. According to the theory, Greenhouse Gases warm the air just slightly above what the ground does, as it warms from absorbing energy from the sun. Parrot Killer Parrot contends that the air does not get warmer than the surface, so the Greenhouse Effect cannot actually work, because you cannot heat something that is already warmer than what you are trying to heat it with. Or, you can't cook coffee over ice. That's an irrelevant argument, if you look at the actual mechanics behind the Greenhouse Effect. Parrot Killer Parrot likes to use the 2nd Law of Thermal Dynamics to defend his claim that you cannot warm something up with ice. That one is hard to argue with, even if you don't know what the 2nd Law of Thermal Dynamics is. 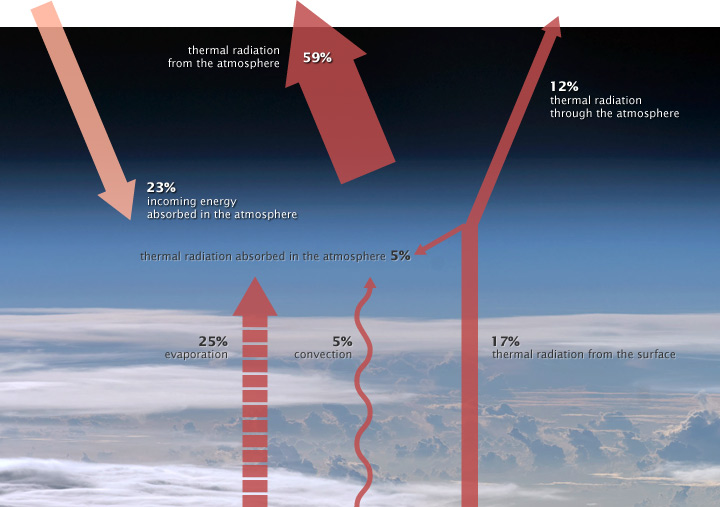 If you want a better look, click below. http://www.climate-debate.com/forum/attachments/atmosphere_energy_balance.jpg That picture shows how energy is released back into space from earth. There are two ways that energy is released. 1) Earth Radiation is generated according to the Stefan-Boltzmann Law. 2) Conduction from Ground to Air and Convection through the air, as heat passes from the planet to space. Notice that the picture shows just 17% of the energy leaving the planet is doing so as "thermal radiation from the surface," 25% leaves through evaporation, and 5% leaves through convection. That means that the majority [30%] of the energy received from sunlight is transferred through the atmosphere to space, as described by the 2nd Law of Thermal Dynamics. Heat moves from hot to cold, at a rate that is dependent on the difference in temperature of the two mediums, and the conduction ability of the coupling. Basically, that is saying that the surface of the earth is cooled quicker by cold air than it is by warm air. If you understand that, as most people do, then you can understand how air that is cooler than the surface, can actually cause the surface to warm. That's because the surface has to either get rid of the thermal energy by warming the air, or it has to get warmer. If the energy transfer out from the surface is faster than the energy transfer to the surface [from the sun], then the surface will cool over time. And if the energy transfer out from the surface is slower than the energy transfer to the surface [from the sun], then the surface will get warmer. If colder air speeds up the transfer of thermal energy from the surface, and warmer air slows down the transfer of thermal energy from the surface, then warming the air slightly above what conduction and convection alone does, then the thermal energy transfer is slowed, and the surface will gradually warm. So in a way, you can warm something with a colder object, just like you warm yourself by putting on a coat, which has no warmth. It simply changes the coupling between you and the air, thus slowing down the rate at which your body can cool itself. You become warmer, even though the coat adds no thermal energy to your body. The same thing is going on with the planet and the atmosphere. ~*~ GreenMan ~*~ https://www.tapatalk.com/groups/leftbehind/index.php |
| 06-09-2017 07:46 | |
| GasGuzzler★★★★★ (2935) |
Litegreen; Put a pan on the stove. Turn on low heat till it reaches max temp. Now tell me what gas...what ANYTHING you can put in that pan to make it hotter without adjusting the heat source? CO2 cannot make the surface hotter than it otherwise would be. CO2 will absorb heat, but as you described, equilibrium will not allow it to go any hotter. Now if you want to argue insulating properties of CO2, well I'd like to see that argument. Edited on 06-09-2017 07:47 |
| 06-09-2017 09:35 | |
| GreenMan★★★☆☆ (661) |
GasGuzzler wrote: Hey Jizz Guzzler, nice to see you back, making fun of my name, and trying to sound intelligent by asking stupid questions. What if I take that pot of water to the ocean? That will get it hotter. But it doesn't have anything to do with how the Greenhouse Effect works. It has to do with increased air pressure, keeping the water from evaporating off as easily as it does at your house out in Iowa. Or I could use a pressure cooker with a lid on it to achieve the same thing, and with even better results. In simple terms, let's take a look at what is going on with the Greenhouse Effect, and I think you will understand. Energy from the Sun strikes the earth. Let's forget about losses due to this, that, and the other thing, for the purpose of this discussion, and say that whatever heat reaches the earth is 100% there is from the Sun. That energy from the Sun raises the temperature of the surface of the earth to 100C, all by itself. We will look at two examples. One example starts with an air temperature of 50C, and the other example starts with an air temperature of 95C. Let's take a look at what happens in the first example, with 50C air. The surface is able to transfer a lot of thermal energy to the air, so very little is penetrating downward. Now let's compare that to what happens in the second example, with 95C air. The surface is not able to transfer as much thermal energy to the air, because the air is almost as warm as it is. So, more thermal energy is penetrating downward, warming the ground below more. Keep in mind, that as long as there is a difference in heat between two molecules, that heat will flow from the hotter to the colder, at a rate that is determined by the difference in heat, and the coupling, according to the 2nd Law of Thermal Dynamics. So if the air is warmer than the ground beneath the surface, then most of the thermal energy goes down, not up. And likewise, if the air is colder than the ground beneath the surface, then most of the heat goes up into the air. So, if you agree that that makes sense, we can move on, and get into why that little bit of heating by gases that absorb infrared radiation [Greenhouse Gases] can actually warm the earth, even though the air doesn't have to get warmer than the planet. All it has to do is get warmer than what the ground's thermal energy is making it. For example, if the ground's thermal energy is causing the air to warm to 90C, then it will cool quicker than it will if an additional 5C is added to it by gases being warmed by earth's radiation. That slows down thermal energy being transferred to the air and that causes the surface to warm, because it has to store more thermal energy. The ground below the surface can store that thermal energy for a while, because of the coupling between it and the surface [which changes as you go deeper]. Consider the ground 30M below the surface, versus ground 3M below the surface. The thermal energy at 3M can move to the surface quicker than the thermal energy at 30M. So the ground at 30M becomes a good storage container for thermal energy. It can, and does hold heat, which we can tell by measuring the temperature with a thermometer. If it couldn't hold thermal energy, then it would be very cold. But it's not cold. It's always about the same temperature as the average annual regional temperature. ~*~ GreenMan ~*~ https://www.tapatalk.com/groups/leftbehind/index.php |
| 06-09-2017 14:29 | |
| GasGuzzler★★★★★ (2935) |
All it has to do is get warmer than what the ground's thermal energy is making it. For example, if the ground's thermal energy is causing the air to warm to 90C, then it will cool quicker than it will if an additional 5C is added to it by gases being warmed by earth's radiation. That slows down thermal energy being transferred to the air and that causes the surface to warm, because it has to store more thermal energy. ??? Seems like a magik 5C. The air and the gas should warm relatively equally if the surface is heating them. Explain this "additional 5C is added to it by gases being warmed by earth's radiation". |
| 06-09-2017 15:25 | |
| GreenMan★★★☆☆ (661) |
GasGuzzler wrote:All it has to do is get warmer than what the ground's thermal energy is making it. For example, if the ground's thermal energy is causing the air to warm to 90C, then it will cool quicker than it will if an additional 5C is added to it by gases being warmed by earth's radiation. That slows down thermal energy being transferred to the air and that causes the surface to warm, because it has to store more thermal energy. It's not magic really, any more than that radio that you listen to on your way to work. Your radio is tuned to a particular radio station by the magic of electrical resonance. You select a frequency to listen to by adjusting the resonant frequency, and whatever is being played on that frequency is what you hear. That 5C [additional heating] comes from the same exact thing, only you can't adjust the frequency that gas molecules resonate at. That is fixed in their molecular makeup. When a beam of light at the resonant frequency of the gas molecule strikes it, the gas molecule stretches and then snaps back, giving off heat as a result. That heat is minute, but it is warmer than the surrounding air, so it warms the surrounding air slightly. During the daytime, there is sometimes an endless supply of light coming off the planet's surface, so that molecule gets to sit there all day long working out, generating his heat, which adds to the heat his neighbor gases get from the surface. So if the surface is warming the air to 20C by itself, the air gets an additional 5C kick from the gases. Actually, it's more like 33C additional warming, at pre-Industrial gas concentration levels. ~*~ GreenMan ~*~ https://www.tapatalk.com/groups/leftbehind/index.php |
| 06-09-2017 20:56 | |
| Into the Night (21599) |
GreenMan wrote: Contextomy. It didn't take any long debate. I have always said that CO2 absorbs infrared light and warms slightly as a result. I have also said that this additional thermal energy in CO2 (only about 1% actually absorbs anything) is just dissipated into the rest of the atmosphere around it. It becomes part of the radiance of Earth. It is warming the atmosphere in just conduction does. The only difference is the use of light to do it. GreenMan wrote: The absorption of light by a gas occurs with ALL gases (and all substances). Many of these, including oxygen and nitrogen, also absorb light in the infrared band. GreenMan wrote: We already have several. Why do you need a new one? If you want to start one, feel free. GreenMan wrote: Not a theory. A vacuous argument. You first have to define 'global warming'. The Sun warms the surface much more than the air. GreenMan wrote: More or less correct. GreenMan wrote: That's called an argument of the Stone. GreenMan wrote: There is only one way for energy to leave the Earth; as radiance. There is nothing to conduct to in space. GreenMan wrote: The thermal form of the 2nd law of thermodynamics. The law does apply to all energy, however. GreenMan wrote: Air is a fluid. It moves. Warm air rises. GreenMan wrote: You can't heat a hotter surface with a colder gas. GreenMan wrote: Did you know that warming the air cools the surface? Did you know that warm air rises? Why do you deny physics? * GreenMan wrote: The energy out is the same as the energy in. GreenMan wrote: WRONG. Warm air rises. The surface is cooled by heating the air. GreenMan wrote: The coat is reducing heat. The air is not an insulator. Putting a coat on a rock will not make the rock any warmer. GreenMan wrote: That is what a coat does. Your body, however, still cools itself. The surface is not a source of energy. YOU are. GreenMan wrote: Because YOU are a source of energy. The surface is not. GreenMan wrote: Nope. The energy to warm the surface is from the Sun. Putting a blanket or coat around Earth would cool the Earth, not warm it. It would keep the Sun out. The Parrot Killer Debunked in my sig. - tmiddles Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit nuclear powered ships do not require nuclear fuel. - Swan While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 06-09-2017 21:19 | |
| Into the Night (21599) |
GasGuzzler wrote: CO2 is not an insulator. It conducts heat about the same as any other gas. Water vapor is not an insulator. It conducts heat BETTER than dry air. Condensed water (clouds) is not an insulator. It conducts heat BETTER than anything else in the sky (except aluminum airplanes). Heat conductivity of various materials is well known and used by engineers when designing cooling systems. If you built a box out of a perfect insulator (if you could find one!), the contents of the box will stay at the same temperature no matter the Sun, how hot or cold the air is, etc. The contents of the box will not get warmer. It will not get colder. The surface is heated by the Sun. Putting an insulator of any kind around the Earth will result in a 'colder' Earth. Since a perfect insulator around the Earth would result in no warming or cooling, there would be no day, and there would be no night. The daytime temperatures would average into the nighttime temperatures. Equatorial temperatures would average into polar temperatures.There would be no seasons. Summer temperatures would merge into winter temperatures. Weather would stop. There would be nothing to drive it. All thermometers would read the same temperature. We would finally know the global temperature...just before we die of the cold. The Parrot Killer Debunked in my sig. - tmiddles Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit nuclear powered ships do not require nuclear fuel. - Swan While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 06-09-2017 21:51 | |
| Into the Night (21599) |
GreenMan wrote:GasGuzzler wrote: No one said anything about water in the pan. However, increased air pressure does generally result in higher temperatures. Temperature is the average kinetic energy of molecules. If there are more molecules per cubic volume, there is more kinetic energy available. Thus, Death Valley at -282ft ASL is generally hotter than nearby Las Vegas at 2000ft ASL or Mt Charleston (near Las Vegas) at 11900 ft ASL, where there is a ski area. GreenMan wrote: Here we go with the same old Magick Bouncing Photon Argument. GreenMan wrote: Gawd I hope not! I HATE it when the ocean boils! GreenMan wrote: Air is not one temperature. Air is a fluid. GreenMan wrote: You can't use 95 deg air to warm a 100 deg surface. GreenMan wrote: 95 deg is NOT warmer than 100 deg. GreenMan wrote: You can't heat a warmer surface with colder air. GreenMan wrote: Thermal energy is measured by temperature, dumbass. GreenMan wrote: The surface is heated by the Sun. The Sun is not underground. The Parrot Killer Debunked in my sig. - tmiddles Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit nuclear powered ships do not require nuclear fuel. - Swan While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 06-09-2017 22:00 | |
| Into the Night (21599) |
GreenMan wrote:GasGuzzler wrote:All it has to do is get warmer than what the ground's thermal energy is making it. For example, if the ground's thermal energy is causing the air to warm to 90C, then it will cool quicker than it will if an additional 5C is added to it by gases being warmed by earth's radiation. That slows down thermal energy being transferred to the air and that causes the surface to warm, because it has to store more thermal energy. I guess radio is magick to you, that's why you used it as an example. GreenMan wrote: Molecules don't have a resonant frequency in that way. GreenMan wrote: No. GreenMan wrote: It does not give off heat at that time. It gains thermal energy. GreenMan wrote: Which is what I've been saying. To warm the surrounding air, it cools again. GreenMan wrote:The supply of light is not endless. The Sun sets at night. Also, not all light results in heat. GreenMan wrote: You can't heat the surface using a colder gas, dumbass. Air rises. It's a fluid. Radiance is how the Earth loses energy to space. Most of that radiance is from the surface itself, but also some from the atmosphere. The Parrot Killer Debunked in my sig. - tmiddles Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit nuclear powered ships do not require nuclear fuel. - Swan While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan Edited on 06-09-2017 22:50 |
| 07-09-2017 08:01 | |
| GreenMan★★★☆☆ (661) |
Into the Night wrote:GreenMan wrote:GasGuzzler wrote:All it has to do is get warmer than what the ground's thermal energy is making it. For example, if the ground's thermal energy is causing the air to warm to 90C, then it will cool quicker than it will if an additional 5C is added to it by gases being warmed by earth's radiation. That slows down thermal energy being transferred to the air and that causes the surface to warm, because it has to store more thermal energy. Radio used to be magic to me, and that was one of the reasons I originally became interested in learning about electrical and electronics. Now it's a part of me. And that is why I used it as an example. When I looked at what was going on at a molecular level, I saw something that I am familiar with. So I related it to what I already knew. You see, that is what intelligent people do. We don't just memorize information, we relate it to other information that we have, and mix it all in. Then we extract the relationships based on that information to help us solve problems. You should try that. Into the Night wrote:GreenMan wrote: Yes it is, brainiac. Oh, it is fixed in the molecule's atomic makeup. You knew what I meant. Into the Night wrote:GreenMan wrote: If it gains thermal energy, then it gives off heat if the thermal energy is greater that the surrounding molecule's thermal energy. And of course it would be, since it is surrounded by Nitrogen, and at the same temperature as Nitrogen before absorbing the radiation. The radiation makes it slightly [I know how you hate the term "tad bit" because of your supposed proper physics mind and all that there] warmer than the surrounding Nitrogen [and Oxygen], so it heats the surrounding Oxygen and Nitrogen. And if you want to get technical about it, it is when the atoms snap back that the molecule gains thermal energy and gives off heat. Into the Night wrote:GreenMan wrote: Well I hear you saying that, but I can't figure out why, since it is so irrelevant that it cools back down. Are you trying to say that it cools back down to where it was before absorbing the radiation? I hope you aren't that idiotic. It settles close to what it was originally, but remember that it just warmed the air around it slightly. So it gets to cool down to just a little bit warmer than it originally was, because its surroundings warmed slightly. Into the Night wrote:GreenMan wrote:The supply of light is not endless. The Sun sets at night. Also, not all light results in heat. The "Sun sets at night," no kidding? That might be why I mentioned twice something about this occurring during the day time. Who said all light results in heat? I think sometimes you might be hitting your pipe a little too much before you start typing. What you got in that thing, anyway? Into the Night wrote:GreenMan wrote: It's a gas, idiot. Gases rise. Fluids fall. And yes, you can warm a surface using a colder gas. Simply raise the temperature of the gas by 5 degrees. The surface will slow down its cooling because of that increase in gas temperature. The net result is that the surface gets warmer, because it cannot release as much thermal energy as it was with a cooler gas temperature. Into the Night wrote: Yes, I get that. I admit that I was thinking that some thermal energy was being released into space, but it isn't, because space has no matter to release it to. The only energy leaving our atmosphere is radiant energy from electromagnetism, proportional to the heat of the planet and the atmosphere. By warming the air, the Greenhouse Gases are causing an increase in radiance of the atmosphere. That's all. The more the Greenhouse Gases warm the air, the more the atmosphere radiates. Not sure why you think that violate the Stefan-Boltzmann Law, or the 2nd Law of Thermal Dynamics. Especially after seeing you go back and forth with your same bull shit. And blocking or absorbing earth's radiation before it gets to space does not directly cause the earth to warm, as you are suggesting by declaring that the total radiation of earth must be viewable from space, or it violates the Stefan-Boltzmann law. That's just deliberate misinformation being presented by you, to try to confuse other people, and make them just as stupid as you are. Misery loves Company. ~*~ GreenMan ~*~ https://www.tapatalk.com/groups/leftbehind/index.php |
| 07-09-2017 21:07 | |
| Into the Night (21599) |
GreenMan wrote:Into the Night wrote:GreenMan wrote:GasGuzzler wrote:All it has to do is get warmer than what the ground's thermal energy is making it. For example, if the ground's thermal energy is causing the air to warm to 90C, then it will cool quicker than it will if an additional 5C is added to it by gases being warmed by earth's radiation. That slows down thermal energy being transferred to the air and that causes the surface to warm, because it has to store more thermal energy. No, it isn't. Molecules and atoms can vibrate at any number of frequencies. GreenMan wrote:Into the Night wrote:GreenMan wrote: If and only if, true. GreenMan wrote: If and only if the other material around the CO2 is colder than the CO2. GreenMan wrote:Into the Night wrote:GreenMan wrote: It is not irrelevant. GreenMan wrote: Once a molecule or atom absorbs a photon, it will not absorb any more light at that frequency until it loses the energy in it. It MUST cool down again before it can absorb another photon at that frequency. GreenMan wrote: Part of quantum physics, dude. It is not idiotic. GreenMan wrote: Air is a fluid. It rises when heated. GreenMan wrote: Air is a fluid. It rises when heated. GreenMan wrote:Into the Night wrote:GreenMan wrote:The supply of light is not endless. The Sun sets at night. Also, not all light results in heat. You seem to have forgotten that. GreenMan wrote:Into the Night wrote:GreenMan wrote: WRONG. Air is a fluid. All gases are a fluid. Warm air rises, just as warm water rises. GreenMan wrote: No, you can't. GreenMan wrote: That takes energy. GreenMan wrote: Only temporarily. Warm air rises. * GreenMan wrote: So you are describing why one day is hot and not another. ...your point? GreenMan wrote:Into the Night wrote: At least you got that right at last. GreenMan wrote: There are no 'greenhouse' gases. ALL of the atmosphere increases radiance when it is warmer. GreenMan wrote: There is no such thing as a 'greenhouse' gas. No gas can warm the Earth. You seem to think that radiance is free. It is not, you know. It takes energy to do that. GreenMan wrote: It does not cause the Earth to warm at all. GreenMan wrote: Now you deny the Stefan-Boltzmann law again. It is not misinformation. It is the way the law works. Anything absorbed by CO2 is simply part of the Earth's radiance. Is it just another way for the surface to cool itself by heating the atmosphere. Air is a fluid. Convection is in play. Warm air rises. Any warming in the atmosphere is simply convected upward and the energy is dissipated as the pressure drops. The Parrot Killer Debunked in my sig. - tmiddles Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit nuclear powered ships do not require nuclear fuel. - Swan While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-09-2017 09:44 | |
| GreenMan★★★☆☆ (661) |
Into the Night wrote:GreenMan wrote:Into the Night wrote:GreenMan wrote:GasGuzzler wrote:All it has to do is get warmer than what the ground's thermal energy is making it. For example, if the ground's thermal energy is causing the air to warm to 90C, then it will cool quicker than it will if an additional 5C is added to it by gases being warmed by earth's radiation. That slows down thermal energy being transferred to the air and that causes the surface to warm, because it has to store more thermal energy. Indeed it does take energy. It gets energy from earth's radiation, by absorbing a little bit of the frequency that it likes. Into the Night wrote:GreenMan wrote: So you are admitting that the surface will slow down its cooling temporarily. Good. That means that you understand that if it is cooling at a slower rate, that the net result is the surface gets warmer, temporarily. Then the warm air rises, and lets the surface get back to cooling at its faster rate. But wait a minute, the gas that replaces the gas that is rising warms up, so the cycle repeats as long as there is sunlight striking the surface. So it's not really temporary. It's continuous, all day long. Into the Night wrote:Uh, no, dumb ass, I'm not describing why one day is hot and not another. I am describing how raising the temperature of gas slows the cooling of the surface, resulting in additional warming of the surface, which results in additional warming of the air, which is called the Greenhouse Effect, which is what is causing Global Warming, which is causing Climate Change. [Yes, I know, those are all make belief terms to you.] Into the Night wrote:GreenMan wrote:Into the Night wrote: Yes, there are greenhouse gases, and that has nothing to do with the increased radiance of the atmosphere, except that the greenhouse gases warm the atmosphere, which does increase the atmosphere's radiance, since it radiates proportional to its temperature. The difference between greenhouse gases and non-greenhouse gases is that greenhouse gases absorb earth radiation and produce heat. The other gases do not. The other gases do get warm, but it's by convection, not directly by radiation. So you are correct that all the atmosphere increases radiance when it is warmer, but it's irrelevant. Into the Night wrote:GreenMan wrote: No, I don't think radiance is free. Well, actually, yes it is free, until the politicians figure out how to tax people for sunshine. Into the Night wrote:GreenMan wrote: No, I am not denying the Stefan-Boltzmann law. I am denying your claim that it gives a damn about whether or not the object's radiation has to make it all the way out to space before it is accounted for by the law. I say the law is satisfied, regardless of where the radiation end up. You think that the radiation has to make it all the way to outer space for some reason. That's insane. Into the Night wrote: Emitting the radiation is what cools the earth. What happens to the radiation has nothing to do with that cooling process. Into the Night wrote: That is clear. What you are misunderstanding, or just ignoring, is that the air that replaces the warmer air that is rising, gets warmed by greenhouse gases, too. It just keeps on happening, as long as there is sunlight striking the surface. ~*~ GreenMan ~*~ https://www.tapatalk.com/groups/leftbehind/index.php |
| 08-09-2017 20:44 | |
| Into the Night (21599) |
GreenMan wrote: Not for long. Warm air rises. The warmer it is, the faster it rises. GreenMan wrote: That cools the surface. The faster the air is replaced (by faster rising warm air) the faster the surface will cool. GreenMan wrote: The ability to absorb light at a certain frequency doesn't mean anything, other than just another way for the surface to cool itself by heating the air. GreenMan wrote: By conduction, actually. Then by convection. GreenMan wrote: No, it's quite relevant. GreenMan wrote: Yes, you are. You are STILL trying to use Holy Gas to absorb light (thus reducing radiance) and heat the Earth at the same time. GreenMan wrote: It does. That is the point of observation for radiance of the Earth. GreenMan wrote: No.It MUST make it all the way to space. Anything absorbed by CO2 heats the air very slightly, just the same as air being heated by contact with the surface. That heating doesn't come for free. It cools the surface to do it. Hot air rises. That rising cools the air. That's what convection does. You are confusing the circulation of air as an energy trap. You are violating the 1st law of thermodynamics with that one. GreenMan wrote: No, that is REQUIRED. Space is the observation point to observe the radiance of the Earth. GreenMan wrote: Violation of the 1st law of thermodynamics. You can't add energy by just circulating air around. If the Earth wasn't rotating, the Sun would stay up all the time. The daytime side would indeed get quite roasty. The nighttime side, however, would get quite cold. The temperature of the Earth doesn't change. As it is, we rotate once in 24 hours. Everyone gets a chance at the Sun for a time. The rotation of the planet simply distributes heat from the day into the night. The temperature of the Earth doesn't change. Holy Gases make no difference. The Parrot Killer Debunked in my sig. - tmiddles Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit nuclear powered ships do not require nuclear fuel. - Swan While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 08-09-2017 21:12 | |
| litesong★★★★★ (2297) |
"old sick silly sleepy sleezy slimy steenkin' filthy vile reprobate rooting (& rotting) racist pukey proud pig AGW denier liar whiner badnight" bluffed: Holy Gases make no difference. Tminimum shows greater upward divergence from past temperatures than Tmaximum does from past temperatures..... a great display of GHG's ability to retard infra-red energy (specially during times the sun is below the horizon) before exiting to space. |
| 09-09-2017 11:00 | |
| GreenMan★★★☆☆ (661) |
Into the Night wrote:GreenMan wrote: That faster rising warm air warms the upper air, which slows the process down. So the amount of cooling the surface is getting slows down, too. That causes the surface temperature to increase. Into the Night wrote:GreenMan wrote: Absorbing surface radiation does not help the surface to cool. Emitting the radiation allows the surface to cool. It doesn't matter what happens to the radiation after that, as far as the surface is concerned, unless that radiation is absorbed by Greenhouse Gases. Then it warms the air a little, which warms the surface a little, by slowing down it's ability to cool. Into the Night wrote:GreenMan wrote: Would you mind telling us what its relevance is then? Or do you really think you get points for just being argumentative? Into the Night wrote:GreenMan wrote: Greenhouse Gases do not reduce the amount of radiation coming from the surface. That is what the Stefan-Boltzman law is about. It doesn't care about what leaves the atmosphere, unless you are talking about the total radiation of earth and its atmosphere. You are just confused about the observation point. Or, more likely, you know the observation point, and are just trying to confuse other people. Into the Night wrote:GreenMan wrote: The Stefan-Boltzmann law does not indicate that the point of observation is from space. Even an idiot would conclude that the law is just defining how much radiation is given off by an object. But I suppose that is too hard for a retard to understand. Into the Night wrote:GreenMan wrote: No it doesn't, idiot. The Stefan-Boltzmann law just says how much radiation a body will emit, relative to its temperature. It doesn't say anything about what happens to the radiation after it leaves the surface, or that it has to make it through the atmosphere. You are just making that up. Into the Night wrote: Emitting radiation is what cools the surface. What happens to that radiation after it is emitted does not cool it even more, dumb ass. That heating does come for free, since it is utilizing infrared energy that is just being thrown away. Into the Night wrote: You do think you are slick, don't you, Pigeon Eater? You are trying to impose an argument on me, just so you can claim that my argument [that I didn't even make, you imposed it on me] is breaking the law. How childish. Grow up little one, the world is waiting. Into the Night wrote:GreenMan wrote: That is obvious. Where else can it be observed from? The problem with your argument is that Stefan-Boltzmann doesn't care about observations, even the slightest. All he cares about is that objects emit what he said they would. I'm pretty sure he isn't hanging around in outer space, checking up on it. Into the Night wrote:GreenMan wrote: That is true. But we aren't just circulating air around, genius. We are also heating the air we are circulating around, with Greenhouse Gases [they are so wunnerful, that some idiots call them Holy Gas, because they can't figure out how they work]. Into the Night wrote: Are you sure about that? Why wouldn't it stay down all the time? I bet it will on the Chinese side. Into the Night wrote: Are you saying that we're all gonna get roasted? Into the Night wrote: And all those Chinese are going to freeze to death, huh. Into the Night wrote: How would you know? You don't even know the temperature of the earth. Into the Night wrote: So what are you trying to do here, lull us into a trance by saying things that are true, so that we will believe you when you tell us your lies? Into the Night wrote: No such thing as Holy Gases, idiot. ~*~ GreenMan ~*~ https://www.tapatalk.com/groups/leftbehind/index.php |
| 09-09-2017 20:01 | |
| Into the Night (21599) |
GreenMan wrote:Into the Night wrote:GreenMan wrote: WRONG. Air is cooled as it rises. You are now trying to violate the 1st law of thermodynamics by adding more energy than the Sun is providing. GreenMan wrote:Into the Night wrote:GreenMan wrote: No, dumbass. GreenMan wrote:Into the Night wrote: Strawman. GreenMan wrote:Into the Night wrote: I don't have to know the temperature of the Earth. It won't change. It requires a change in energy to change. That energy is the Sun. The only way for the Earth to warm is to increase the output of the Sun. GreenMan wrote:Into the Night wrote: Argument of ridicule. GreenMan wrote:Into the Night wrote: There sure is. You worship them, being part of the Church of Global Warming. The Parrot Killer Debunked in my sig. - tmiddles Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit nuclear powered ships do not require nuclear fuel. - Swan While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 10-09-2017 10:54 | |
| GreenMan★★★☆☆ (661) |
Into the Night wrote: I stripped out everything else, because most of it is just the round and round we go about the same things. This part is worth discussing though, since it shows what you are failing to understand. Let's take this most basic example. Put a coat on. Does that make you warmer? According to your argument, it shouldn't, because the coat does not add any energy to the source of heat, which is your body. Yet is does warm you up. Even though the coat is colder than your body, it has this almost magical ability to warm you up. That seems to be a violation of the 1st and 2nd Law of Thermodynamics, doesn't it? According to you it is. How can it be? We aren't increasing any energy, yet we feel warmer when we put that coat on. Oh, I know why. It's because we slowed down the transfer of thermal energy from out body to the air, when we put the coat on. That allowed our body to store a little more of that thermal energy it was generating. Wow, we just increased the temperature of a body, with something that was colder than the body, without increasing the amount of energy the body is generating. Remarkable! That appears to be in violation of the 1st and 2nd Law of Thermodynamics, doesn't it? And not only that, we totally blew the Stefan-Boltzmann law away, since we can no longer view the body's radiation from space. How can that be? Isn't the body still radiating based on it's temperature? That must be a violation of the Stefan-Boltzmann Law, because according to you, the body's radiation must make it all the way to space to be accounted for. But it's not making it that far, since the coat is blocking it almost completely, unless it's a transparent coat. According to you, we should explode or something, after putting a coat on, due to violating the Stefan-Boltzmann law and the 1st and 2nd Law of Thermodynamics. So you must be wrong, because we do not blow up when we put a coat on. See how that works, I twisted what you were saying around, and spit it back out at you as if it was really what you said. But you never said we would actually blow up for violating those laws, did you? But anyway, those Holy Gases that you like to call them. Well they do the same thing as that coat does. The slow down the rate of thermal energy transfer, by raising the temperature of the air. That slows down the rate of cooling the surface can do. And that, my feathered friend, causes the surface to store a little more thermal energy than it normally does. And we can measure that additional heat with a thing we call a thermometer. We can even place those thermometers around the world, and get a pretty good feel for what the whole world's temperature is. And we can write that temperature average down, and compare it to the previous years average. Yes, I know, that isn't allowed in your make believe world, but we are going to do it anyway, because the rest of us don't have to live with you in your make believe world. ~*~ GreenMan ~*~ https://www.tapatalk.com/groups/leftbehind/index.php |
| 10-09-2017 19:16 | |
| Into the Night (21599) |
GreenMan wrote:Into the Night wrote: I stripped out the remaining part of your repetition of the Magick Blanket argument since it consumes much bulk to say this same thing in different ways. Yes. The coat makes you warmer. Your body temperature is 98 deg F. The outside air is considerably cooler than that much of the time. You aren't dead. I can tell that by your continuing to make stupid arguments that have already been answered. This means you will consume energy to KEEP your body temperature 98 deg F. I assume you eat, and drink, right? The ground does not consume energy to keep itself warm. Neither does the air. Putting a coat on a rock or even a dead body won't keep it warm. CO2 has no ability to insulate. It conducts heat about the same as any other gas. The atmosphere is open. It is a fluid. It moves. Further, radiance from the Earth covers a broad swath of infrared frequencies. CO2 is only sensitive to a narrow band of it. What DOES get absorbed is simply another way for the surface to heat the air, much like conduction. Radiance of the Earth doesn't change, because the CO2 is now radiating that energy instead of the surface. The CO2 further dissipates energy into surrounding air, and convection causes warm air to rise, cooling it. Now before you start into the Magick Bouncing Photon argument again, realize that CO2 is NOT capable of rewarming the surface. It is colder than the surface. Taking energy from CO2 for such a 'purpose' would necessarily reduce the radiance of the Earth just so you can warm the surface (increasing the temperature of the whole Earth) and violate the Stefan-Boltzmann law. Now...since you've decided to ignore the answers in this post, which do you want to go around again on? The Magick Blanket argument or the Magick Bouncing Photon argument? How about trying to define 'global warming' or 'climate change' without using circular definitions? The Parrot Killer Debunked in my sig. - tmiddles Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit nuclear powered ships do not require nuclear fuel. - Swan While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan Edited on 10-09-2017 19:17 |
Join the debate Greenhouse Effect is Real:
Related content
| Threads | Replies | Last post |
| Real Perspective on Warming - NASA Data | 16 | 26-04-2024 06:48 |
| The "radiative Greenhouse effect" does not exist | 145 | 24-04-2024 02:48 |
| What a "REAL" American Brings to the Table | 3 | 05-12-2023 01:14 |
| None Of You Know The Real Intend, Purpose Of Climate Change Issue On The Media | 7 | 04-12-2023 04:02 |
| 'Greenhouse' Effect? | 49 | 30-11-2023 06:45 |