| The Complexity of Climate Change21-03-2017 16:32 |
James_★★★★★
(2238) |
Hi All,
Am new here but have my own thoughts based on research that I have done. And so everyone will understand my position there is what NOAA has posted on it's website and it seems or appears to be that they are quoting the IPCC.
Why this specific information is something that I find interesting is because I have been pursuing an experiment for the last couple of years that would help scientists to understand the link in Atmospheric Chemistry that is missing.
And as I think everyone knows the current belief is that Atmospheric Forcing does not occur in the tropopause. I think successfully demonstrating this would change the discussion about climate change.
Carbon dioxide (CO2), nitrous oxide (N2O), and methane (CH4) are each important to climate forcing and to the levels of stratospheric ozone (see Chapter 2). In terms of the globally averaged ozone column, additional N2O leads to lower ozone levels, whereas additional CO2 and CH4 lead to higher ozone levels. Ozone depletion to date would have been greater if not for the historical increases in CO2 and CH4. The net impact on ozone recovery and future levels of stratospheric ozone thus depends on the future abundances of these gases. For many of the scenarios used in the most recent Intergovernmental Panel on Climate Change (IPCC) Assessment (IPCC, 2013), global ozone will increase to above pre-1980 levels due to future trends in the gases. Latitudinal and altitudinal responses are expected to vary. Note that scenarios used in IPCC consider a future with all three major greenhouse gases increasing and thus it is important to assess the net balance of these perturbations on stratospheric ozone.
https://www.esrl.noaa.gov/csd/assessments/ozone/2014/summary/ch5.html
This part is especially interesting because fluorocarbons were banned in 1987 because of how much they damaged the ozone layer.
>> The net impact on ozone recovery and future levels of stratospheric ozone thus depends on the future abundances of these gases. For many of the scenarios used in the most recent Intergovernmental Panel on Climate Change (IPCC) Assessment (IPCC, 2013), global ozone will increase to above pre-1980 levels due to future trends in the gases. <<
edited to add; this link is to some of the reasons we need to protect the ozone layer. https://www.epa.gov/ozone-layer-protection/health-and-environmental-effects-ozone-layer-depletion
Edited on 21-03-2017 17:09 |
| 21-03-2017 17:53 |
still learning★★☆☆☆
(244) |
James_ wrote:
This part is especially interesting because fluorocarbons were banned in 1987 because of how much they damaged the ozone layer.
As per the Montreal Protocol, the production of some chlorofluorocarbons was phased out after the signing of the protocol. Some phaseout of hydrochloroflouorocarbons extended to 2030.
Hydroflourocarbons, not ozone depleting, were not originally included in the Montreal Protocol, but were later included in an amendment.
see https://en.wikipedia.org/wiki/Montreal_Protocol
What does the ozone layer have to do with "Atmospheric Forcing?" |
| 21-03-2017 18:34 |
Into the Night ★★★★★ ★★★★★
(21598) |
Ozone's biggest threat is ozone. The stuff self destructs to oxygen after a few hours. That process is aided by UV-C sunlight at the top of the stratosphere.
The ozone layer is depleted every night. It is rebuilt every day. As long as you have sunlight and oxygen, you WILL have ozone. We couldn't destroy the ozone layer even if we wanted to.
The CFC ban involved R-12 refrigerant, a substance that Dow Chemical was losing the patent on. They created the 'Ozone Scare' to force everyone onto R-134a, the new substance they just got a patent for.
It was Dow Chemical using the government and the news media to maintain its monopoly.
CFC's are heavier than air. They are like propane. They tend to go down, not up. They are also inert. As a CFC, they will not affect ozone. They must be broken up first.
The component supposedly that DOES affect the ozone layer in CFC's is chlorine. This is a VERY reactive substance. Free chlorine will react with something else before it gets anywhere near the ozone layer. The only 'crisis' was a politically manufactured one.
NOAA basically copies the IPCC graph. They are not capable of producing a graph of global temperatures. No one is. We don't have sufficient instrumentation to produce such a graph. We never have. |
| 21-03-2017 18:54 |
spot★★★★☆
(1323) |
Into the Night wrote:
Ozone's biggest threat is ozone. The stuff self destructs to oxygen after a few hours. That process is aided by UV-C sunlight at the top of the stratosphere.
The ozone layer is depleted every night. It is rebuilt every day. As long as you have sunlight and oxygen, you WILL have ozone. We couldn't destroy the ozone layer even if we wanted to.
The CFC ban involved R-12 refrigerant, a substance that Dow Chemical was losing the patent on. They created the 'Ozone Scare' to force everyone onto R-134a, the new substance they just got a patent for.
It was Dow Chemical using the government and the news media to maintain its monopoly.
CFC's are heavier than air. They are like propane. They tend to go down, not up. They are also inert. As a CFC, they will not affect ozone. They must be broken up first.
The component supposedly that DOES affect the ozone layer in CFC's is chlorine. This is a VERY reactive substance. Free chlorine will react with something else before it gets anywhere near the ozone layer. The only 'crisis' was a politically manufactured one.
NOAA basically copies the IPCC graph. They are not capable of producing a graph of global temperatures. No one is. We don't have sufficient instrumentation to produce such a graph. We never have.
That's a fantastic post, I of course mean fantastic in the sense that it's a product of fantasy.
IBdaMann wrote:
"Air" is not a body in and of itself. Ergo it is not a blackbody.
Planck's law describes the spectral density of electromagnetic radiation emitted by a black body in thermal equilibrium at a given temperature T. |
| 21-03-2017 21:32 |
Wake★★★★★
(4034) |
Into the Night wrote:
Ozone's biggest threat is ozone. The stuff self destructs to oxygen after a few hours. That process is aided by UV-C sunlight at the top of the stratosphere.
The ozone layer is depleted every night. It is rebuilt every day. As long as you have sunlight and oxygen, you WILL have ozone. We couldn't destroy the ozone layer even if we wanted to.
The CFC ban involved R-12 refrigerant, a substance that Dow Chemical was losing the patent on. They created the 'Ozone Scare' to force everyone onto R-134a, the new substance they just got a patent for.
It was Dow Chemical using the government and the news media to maintain its monopoly.
CFC's are heavier than air. They are like propane. They tend to go down, not up. They are also inert. As a CFC, they will not affect ozone. They must be broken up first.
The component supposedly that DOES affect the ozone layer in CFC's is chlorine. This is a VERY reactive substance. Free chlorine will react with something else before it gets anywhere near the ozone layer. The only 'crisis' was a politically manufactured one.
NOAA basically copies the IPCC graph. They are not capable of producing a graph of global temperatures. No one is. We don't have sufficient instrumentation to produce such a graph. We never have.
To agree with your statement - where is the ozone layer the least? At the south pole where there was never any chlorofluorocarbons to supposedly break them down.
I'm not sure that one copied the other. But they were both working with faulty data sets. And it has been politically incorrect to change anything. |
|
| 21-03-2017 21:39 |
Wake★★★★★
(4034) |
spot wrote:
Into the Night wrote:
Ozone's biggest threat is ozone. The stuff self destructs to oxygen after a few hours. That process is aided by UV-C sunlight at the top of the stratosphere.
The ozone layer is depleted every night. It is rebuilt every day. As long as you have sunlight and oxygen, you WILL have ozone. We couldn't destroy the ozone layer even if we wanted to.
The CFC ban involved R-12 refrigerant, a substance that Dow Chemical was losing the patent on. They created the 'Ozone Scare' to force everyone onto R-134a, the new substance they just got a patent for.
It was Dow Chemical using the government and the news media to maintain its monopoly.
CFC's are heavier than air. They are like propane. They tend to go down, not up. They are also inert. As a CFC, they will not affect ozone. They must be broken up first.
The component supposedly that DOES affect the ozone layer in CFC's is chlorine. This is a VERY reactive substance. Free chlorine will react with something else before it gets anywhere near the ozone layer. The only 'crisis' was a politically manufactured one.
NOAA basically copies the IPCC graph. They are not capable of producing a graph of global temperatures. No one is. We don't have sufficient instrumentation to produce such a graph. We never have.
That's a fantastic post, I of course mean fantastic in the sense that it's a product of fantasy.
You simply cannot contain your ignorance can you?
"One of the founding fathers of the French ecological movement and a former minister for the prevention of major natural and technological risks, Tazieff is well qualified to talk about environmental issues. He has been adviser to most of France's environment ministers over the past decade. Despite this he asserts that Green parties are running a "campaign of deliberate, untruthful scaremongering," and the imaginary problems they espouse have led to millions of pounds being directed towards "environmental windmills" rather than the real threats of pollution. It seemed strange to Tazieff that an ozone hole situated above the Antarctic was blamed on CFC gases, when most deodorants were sprayed in the northern hemisphere. He was surprised to discover an article in the 1950 Annals of Geophysics reporting the existence of ozone holes above Norway in 1926 - years before CFC's were even dreamt of - and was astounded to find that the hole above the Antarctic was not the recent phenomenon ecologists claimed it to be. It was actually discovered as far back as 1957, he says, by the English scientist, Gordon Dobson, but it was only in the mid-eighties that satellite photos began to highlight it in a rather spectacular way.
Tazieff believes that these dramatic images have been used to hoodwink the public. He believes that the hole is due to the low levels of ultraviolet rays (which are necessary to produce ozone) over the Antarctic at the end of the year, and that the large and swift movements of air masses around the continent also play their part. On September 5, 1987, there was a relatively large reduction of 0.1 per cent in the levels of ozone over a surface of three million square kilometres near the Palmer peninsula in the Antarctic. Tazieff is convinced there is no way that the CFCs could have broken down so much ozone in such a short space of time." |
| 21-03-2017 23:12 |
Into the Night ★★★★★ ★★★★★
(21598) |
Wake wrote:
spot wrote:
Into the Night wrote:
Ozone's biggest threat is ozone. The stuff self destructs to oxygen after a few hours. That process is aided by UV-C sunlight at the top of the stratosphere.
The ozone layer is depleted every night. It is rebuilt every day. As long as you have sunlight and oxygen, you WILL have ozone. We couldn't destroy the ozone layer even if we wanted to.
The CFC ban involved R-12 refrigerant, a substance that Dow Chemical was losing the patent on. They created the 'Ozone Scare' to force everyone onto R-134a, the new substance they just got a patent for.
It was Dow Chemical using the government and the news media to maintain its monopoly.
CFC's are heavier than air. They are like propane. They tend to go down, not up. They are also inert. As a CFC, they will not affect ozone. They must be broken up first.
The component supposedly that DOES affect the ozone layer in CFC's is chlorine. This is a VERY reactive substance. Free chlorine will react with something else before it gets anywhere near the ozone layer. The only 'crisis' was a politically manufactured one.
NOAA basically copies the IPCC graph. They are not capable of producing a graph of global temperatures. No one is. We don't have sufficient instrumentation to produce such a graph. We never have.
That's a fantastic post, I of course mean fantastic in the sense that it's a product of fantasy.
You simply cannot contain your ignorance can you?
"One of the founding fathers of the French ecological movement and a former minister for the prevention of major natural and technological risks, Tazieff is well qualified to talk about environmental issues. He has been adviser to most of France's environment ministers over the past decade. Despite this he asserts that Green parties are running a "campaign of deliberate, untruthful scaremongering," and the imaginary problems they espouse have led to millions of pounds being directed towards "environmental windmills" rather than the real threats of pollution. It seemed strange to Tazieff that an ozone hole situated above the Antarctic was blamed on CFC gases, when most deodorants were sprayed in the northern hemisphere. He was surprised to discover an article in the 1950 Annals of Geophysics reporting the existence of ozone holes above Norway in 1926 - years before CFC's were even dreamt of - and was astounded to find that the hole above the Antarctic was not the recent phenomenon ecologists claimed it to be. It was actually discovered as far back as 1957, he says, by the English scientist, Gordon Dobson, but it was only in the mid-eighties that satellite photos began to highlight it in a rather spectacular way.
Tazieff believes that these dramatic images have been used to hoodwink the public. He believes that the hole is due to the low levels of ultraviolet rays (which are necessary to produce ozone) over the Antarctic at the end of the year, and that the large and swift movements of air masses around the continent also play their part. On September 5, 1987, there was a relatively large reduction of 0.1 per cent in the levels of ozone over a surface of three million square kilometres near the Palmer peninsula in the Antarctic. Tazieff is convinced there is no way that the CFCs could have broken down so much ozone in such a short space of time."
If you expose ozone to CFC's, nothing happens.
If you break up the CFC's into chlorine and fluorocarbon, the chlorine reacts to destroy ozone. It also reacts with practically everything else. The fluorocarbon component remains stable and doesn't take part in the reaction.
The biggest 'danger' to ozone is ozone itself. It naturally decomposes back to oxygen in a few hours once the energy producing it has been removed. Exposure to UV-C also destroys ozone.
Ozone is created at the bottom of the stratosphere (which is why the tropopause is so cold) by UV-B and UV-A action on oxygen. By this altitude, UV-C has been completely absorbed by the destruction of ozone at the top of the stratosphere (which is why it gets hotter as you rise in the stratosphere).
At night, no Sun, no ozone production or destruction. Decay begins immediately and progresses throughout the night. The ozone layer is rebuilt again when the Sun rises. This delay is why you burn in the late morning (you don't feel that for a few hours after the actual burn, which makes it seem like you burn in the afternoon).
As long as you have sunlight and oxygen, you WILL have ozone.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan
Edited on 21-03-2017 23:16 |
| 21-03-2017 23:44 |
Surface Detail★★★★☆
(1673) |
Into the Night wrote:
Wake wrote:
spot wrote:
Into the Night wrote:
Ozone's biggest threat is ozone. The stuff self destructs to oxygen after a few hours. That process is aided by UV-C sunlight at the top of the stratosphere.
The ozone layer is depleted every night. It is rebuilt every day. As long as you have sunlight and oxygen, you WILL have ozone. We couldn't destroy the ozone layer even if we wanted to.
The CFC ban involved R-12 refrigerant, a substance that Dow Chemical was losing the patent on. They created the 'Ozone Scare' to force everyone onto R-134a, the new substance they just got a patent for.
It was Dow Chemical using the government and the news media to maintain its monopoly.
CFC's are heavier than air. They are like propane. They tend to go down, not up. They are also inert. As a CFC, they will not affect ozone. They must be broken up first.
The component supposedly that DOES affect the ozone layer in CFC's is chlorine. This is a VERY reactive substance. Free chlorine will react with something else before it gets anywhere near the ozone layer. The only 'crisis' was a politically manufactured one.
NOAA basically copies the IPCC graph. They are not capable of producing a graph of global temperatures. No one is. We don't have sufficient instrumentation to produce such a graph. We never have.
That's a fantastic post, I of course mean fantastic in the sense that it's a product of fantasy.
You simply cannot contain your ignorance can you?
"One of the founding fathers of the French ecological movement and a former minister for the prevention of major natural and technological risks, Tazieff is well qualified to talk about environmental issues. He has been adviser to most of France's environment ministers over the past decade. Despite this he asserts that Green parties are running a "campaign of deliberate, untruthful scaremongering," and the imaginary problems they espouse have led to millions of pounds being directed towards "environmental windmills" rather than the real threats of pollution. It seemed strange to Tazieff that an ozone hole situated above the Antarctic was blamed on CFC gases, when most deodorants were sprayed in the northern hemisphere. He was surprised to discover an article in the 1950 Annals of Geophysics reporting the existence of ozone holes above Norway in 1926 - years before CFC's were even dreamt of - and was astounded to find that the hole above the Antarctic was not the recent phenomenon ecologists claimed it to be. It was actually discovered as far back as 1957, he says, by the English scientist, Gordon Dobson, but it was only in the mid-eighties that satellite photos began to highlight it in a rather spectacular way.
Tazieff believes that these dramatic images have been used to hoodwink the public. He believes that the hole is due to the low levels of ultraviolet rays (which are necessary to produce ozone) over the Antarctic at the end of the year, and that the large and swift movements of air masses around the continent also play their part. On September 5, 1987, there was a relatively large reduction of 0.1 per cent in the levels of ozone over a surface of three million square kilometres near the Palmer peninsula in the Antarctic. Tazieff is convinced there is no way that the CFCs could have broken down so much ozone in such a short space of time."
If you expose ozone to CFC's, nothing happens.
If you break up the CFC's into chlorine and fluorocarbon, the chlorine reacts to destroy ozone. It also reacts with practically everything else. The fluorocarbon component remains stable and doesn't take part in the reaction.
The biggest 'danger' to ozone is ozone itself. It naturally decomposes back to oxygen in a few hours once the energy producing it has been removed. Exposure to UV-C also destroys ozone.
Ozone is created at the bottom of the stratosphere (which is why the tropopause is so cold) by UV-B and UV-A action on oxygen. By this altitude, UV-C has been completely absorbed by the destruction of ozone at the top of the stratosphere (which is why it gets hotter as you rise in the stratosphere).
At night, no Sun, no ozone production or destruction. Decay begins immediately and progresses throughout the night. The ozone layer is rebuilt again when the Sun rises. This delay is why you burn in the late morning (you don't feel that for a few hours after the actual burn, which makes it seem like you burn in the afternoon).
As long as you have sunlight and oxygen, you WILL have ozone.
According to this site:
http://www.lenntech.com/library/ozone/decomposition/ozone-decomposition.htm
the half-life of ozone in air at a temperature of -50°C (the approximate temperature of the ozone layer) is 3 months, not a few hours.
This actual fact rather scuppers your argument, does it not? |
| 22-03-2017 00:08 |
Wake★★★★★
(4034) |
Surface Detail wrote:
According to this site:
http://www.lenntech.com/library/ozone/decomposition/ozone-decomposition.htm
the half-life of ozone in air at a temperature of -50°C (the approximate temperature of the ozone layer) is 3 months, not a few hours.
This actual fact rather scuppers your argument, does it not?
And all you have to look at is wikipedia to see that the boiling point is -112C and that the vapor pressure is 20C meaning that
O3 is so unstable that it would naturally decay O + O3 = 2 O2 in a day or so particularly in angled sunlight where UV is available. And in some case mere minutes.
If you rely upon computer modeling instead of actual measurements you keep getting bit in the butt.
Rather than arguing about inconsequential things surely you could put your mind to more important things. What do you do for a living? Since you are on the web continually I assume you are either retired or seeking work.
What is your real skill set? I may razz hell out of you but I certainly do not think of anything above lint picker as beneath people's respect. |
| 22-03-2017 00:31 |
Surface Detail★★★★☆
(1673) |
Wake wrote:
Surface Detail wrote:
According to this site:
http://www.lenntech.com/library/ozone/decomposition/ozone-decomposition.htm
the half-life of ozone in air at a temperature of -50°C (the approximate temperature of the ozone layer) is 3 months, not a few hours.
This actual fact rather scuppers your argument, does it not?
And all you have to look at is wikipedia to see that the boiling point is -112C and that the vapor pressure is 20C meaning that
O3 is so unstable that it would naturally decay O + O3 = 2 O2 in a day or so particularly in angled sunlight where UV is available. And in some case mere minutes.
If you rely upon computer modeling instead of actual measurements you keep getting bit in the butt.
Rather than arguing about inconsequential things surely you could put your mind to more important things. What do you do for a living? Since you are on the web continually I assume you are either retired or seeking work.
What is your real skill set? I may razz hell out of you but I certainly do not think of anything above lint picker as beneath people's respect.
Your post makes no sense whatsoever. The vapour pressure of ozone has nothing to do with its stability. And how can the vapour pressure be 20C? Those aren't even the correct units. And who mentioned computer modelling?
As for time spent on the web:
http://www.climate-debate.com/topusers.php
Most active users
Climate-Debate.com > Users > Most active users
# / User / Posts / Posts/day
4 / Surface Detail / 1186 / 1.38
12 / Wake / 513 / 10.68
Somehow, I still manage to earn a living while making an average of one and a bit posts per day to this site. And you clearly spend a lot more time here than I do. |
| 22-03-2017 00:42 |
Wake★★★★★
(4034) |
Surface Detail wrote:
Wake wrote:
Surface Detail wrote:
According to this site:
http://www.lenntech.com/library/ozone/decomposition/ozone-decomposition.htm
the half-life of ozone in air at a temperature of -50°C (the approximate temperature of the ozone layer) is 3 months, not a few hours.
This actual fact rather scuppers your argument, does it not?
And all you have to look at is wikipedia to see that the boiling point is -112C and that the vapor pressure is 20C meaning that
O3 is so unstable that it would naturally decay O + O3 = 2 O2 in a day or so particularly in angled sunlight where UV is available. And in some case mere minutes.
If you rely upon computer modeling instead of actual measurements you keep getting bit in the butt.
Rather than arguing about inconsequential things surely you could put your mind to more important things. What do you do for a living? Since you are on the web continually I assume you are either retired or seeking work.
What is your real skill set? I may razz hell out of you but I certainly do not think of anything above lint picker as beneath people's respect.
Your post makes no sense whatsoever. The vapour pressure of ozone has nothing to do with its stability. And how can the vapour pressure be 20C? Those aren't even the correct units. And who mentioned computer modelling?
As for time spent on the web:
http://www.climate-debate.com/topusers.php
Most active users
Climate-Debate.com > Users > Most active users
# / User / Posts / Posts/day
4 / Surface Detail / 1186 / 1.38
12 / Wake / 513 / 10.68
Somehow, I still manage to earn a living while making an average of one and a bit posts per day to this site. And you clearly spend a lot more time here than I do.
But I am retired and according to you you are not. Likely story. Are you Cubical Crow? If the boss catches you you're unemployed immediately? And in the last week I put in over 100 miles on my bike besides and cooked fine dinners for my wife when she gets home from babysitting the grandchildren. And I build my own bikes from bare frames. And I'm still a life member in my local yacht club that was founded under the aegis of the President of the US in 1908. And you won't see me for a couple of weeks as I go off on a Norwegian cruise liner.
The market dropped 200 points today but I'm still worth a million bucks. What are you worth? |
| 22-03-2017 01:03 |
GasGuzzler★★★★★
(2933) |
I'm worth a million dollars also...but only if I die. |
| 22-03-2017 01:36 |
Wake★★★★★
(4034) |
GasGuzzler wrote:
I'm worth a million dollars also...but only if I die.
Raise the amount to double that. A million isn't enough to survive on anymore. |
| 22-03-2017 16:06 |
James_★★★★★
(2238) |
still learning wrote:
James_ wrote:
This part is especially interesting because fluorocarbons were banned in 1987 because of how much they damaged the ozone layer.
As per the Montreal Protocol, the production of some chlorofluorocarbons was phased out after the signing of the protocol. Some phaseout of hydrochloroflouorocarbons extended to 2030.
Hydroflourocarbons, not ozone depleting, were not originally included in the Montreal Protocol, but were later included in an amendment.
see https://en.wikipedia.org/wiki/Montreal_Protocol
What does the ozone layer have to do with "Atmospheric Forcing?"
still learning,
With Atmospheric Forcing, if demonstrated would help to explain how CO2 and CH4's historic levels are helping to prevent further depletion of the ozone layer.
And this is according to the IPCC itself. This creates a slight problem because the IPCC supports the belief that the ozone layer will recover to pre-1980 levels. A part of this belief might be based on the assumption that CO2 levels will keep rising. And it hasn't been discussed what will happen if CO2 levels are lowered and we lose our ozone layer.
@All,
As for the half life of ozone, it seems all answers are based on bad ozone which is in the air. This one link shows that in olive oil that ozone will last for 2 years. Why I find this interesting is because if ozone 's half life was only 30 minutes then they would be saying that cfc's and chlorine-bromide gases would be keeping stratospheric ozone from occurring.
And the 2nd link shows where Carbon tetrachloride (CCl4) which is a banned CFC which is found in Antarctica when there is no known source for it.
And I guess if I can show that Atmospheric Forcing does happen then maybe testing in the stratosphere itself can be done to verify ozone's half life in that specific environment.
Jim
http://drsozone.com/ozone-info/what-is-ozone-2/
https://www.nasa.gov/press/2014/august/ozone-depleting-compound-persists-nasa-research-shows/
edited to add;
quoting the EPA
Most atmospheric ozone is concentrated in a layer in the stratosphere, about 9 to 18 miles (15 to 30 km) above the Earth's surface (see the figure below). Ozone is a molecule that contains three oxygen atoms. At any given time, ozone molecules are constantly formed and destroyed in the stratosphere. The total amount has remained relatively stable during the decades that it has been measured.
https://www.epa.gov/ozone-layer-protection/basic-ozone-layer-science
I think as for myself I will continue to pursue my experiment and question the half life of stratospheric ozone. There is research that suggests that stratospheric ozone has a much longer half life.
Edited on 22-03-2017 16:14 |
| 22-03-2017 16:55 |
Wake★★★★★
(4034) |
James_ wrote: I think as for myself I will continue to pursue my experiment and question the half life of stratospheric ozone. There is research that suggests that stratospheric ozone has a much longer half life.
One direction of study might be to calculate the actual temperature of ozone molecules in the upper atmosphere. Remember that they are directly heated by UV from the Sun's emissions and hence tend to have, individually a very high temperature. This hastens to half life a great deal and my guess is the half life in the ozone region to be in seconds rather than in hours. But since it is not only destroyed but formed as well it is difficult to measure and must be calculated. |
|
| 22-03-2017 18:32 |
Surface Detail★★★★☆
(1673) |
Wake wrote:
James_ wrote: I think as for myself I will continue to pursue my experiment and question the half life of stratospheric ozone. There is research that suggests that stratospheric ozone has a much longer half life.
One direction of study might be to calculate the actual temperature of ozone molecules in the upper atmosphere. Remember that they are directly heated by UV from the Sun's emissions and hence tend to have, individually a very high temperature. This hastens to half life a great deal and my guess is the half life in the ozone region to be in seconds rather than in hours. But since it is not only destroyed but formed as well it is difficult to measure and must be calculated.
Wrong.
The pressure and temperature in the ozone layer are about 50 mbar and -50C respectively. Plugging those figures together with a mean molecular diameter of 2.5 nm and mass of 29 amu for air into the site below gives a collision frequency of about 2 x 10^6 per second. This means that the ozone molecules reach thermal equilibrium with the surrounding molecules within a few microseconds. At -50C, the half-life of the ozone will be about 3 months.
http://hyperphysics.phy-astr.gsu.edu/hbase/Kinetic/frecol.html#c1 |
| 22-03-2017 20:29 |
Wake★★★★★
(4034) |
Surface Detail wrote:
Wake wrote:
James_ wrote: I think as for myself I will continue to pursue my experiment and question the half life of stratospheric ozone. There is research that suggests that stratospheric ozone has a much longer half life.
One direction of study might be to calculate the actual temperature of ozone molecules in the upper atmosphere. Remember that they are directly heated by UV from the Sun's emissions and hence tend to have, individually a very high temperature. This hastens to half life a great deal and my guess is the half life in the ozone region to be in seconds rather than in hours. But since it is not only destroyed but formed as well it is difficult to measure and must be calculated.
Wrong.
The pressure and temperature in the ozone layer are about 50 mbar and -50C respectively. Plugging those figures together with a mean molecular diameter of 2.5 nm and mass of 29 amu for air into the site below gives a collision frequency of about 2 x 10^6 per second. This means that the ozone molecules reach thermal equilibrium with the surrounding molecules within a few microseconds. At -50C, the half-life of the ozone will be about 3 months.
http://hyperphysics.phy-astr.gsu.edu/hbase/Kinetic/frecol.html#c1
You continue to make it clear that you are unaware of science. So perhaps you should stay out of conversations that are not directed at you. |
| 22-03-2017 20:40 |
Surface Detail★★★★☆
(1673) |
Wake wrote:
Surface Detail wrote:
Wake wrote:
James_ wrote: I think as for myself I will continue to pursue my experiment and question the half life of stratospheric ozone. There is research that suggests that stratospheric ozone has a much longer half life.
One direction of study might be to calculate the actual temperature of ozone molecules in the upper atmosphere. Remember that they are directly heated by UV from the Sun's emissions and hence tend to have, individually a very high temperature. This hastens to half life a great deal and my guess is the half life in the ozone region to be in seconds rather than in hours. But since it is not only destroyed but formed as well it is difficult to measure and must be calculated.
Wrong.
The pressure and temperature in the ozone layer are about 50 mbar and -50C respectively. Plugging those figures together with a mean molecular diameter of 2.5 nm and mass of 29 amu for air into the site below gives a collision frequency of about 2 x 10^6 per second. This means that the ozone molecules reach thermal equilibrium with the surrounding molecules within a few microseconds. At -50C, the half-life of the ozone will be about 3 months.
http://hyperphysics.phy-astr.gsu.edu/hbase/Kinetic/frecol.html#c1
You continue to make it clear that you are unaware of science. So perhaps you should stay out of conversations that are not directed at you.
I won't be bullied into silence by you or anyone else. While you keep writing lies and nonsense, I'll keep pointing it out.
Now, do you understand what I wrote? Because the ozone molecules collide with other molecules about a million times per second, they almost immediately pass on to neighbouring molecules the energy they gain from absorbing UV photons. They cannot individually have a very high temperature, as you claim. |
| 22-03-2017 21:33 |
James_★★★★★
(2238) |
Wake wrote:
James_ wrote: I think as for myself I will continue to pursue my experiment and question the half life of stratospheric ozone. There is research that suggests that stratospheric ozone has a much longer half life.
One direction of study might be to calculate the actual temperature of ozone molecules in the upper atmosphere. Remember that they are directly heated by UV from the Sun's emissions and hence tend to have, individually a very high temperature. This hastens to half life a great deal and my guess is the half life in the ozone region to be in seconds rather than in hours. But since it is not only destroyed but formed as well it is difficult to measure and must be calculated.
Wake,
One reason for the experiment is to see if there is atmospheric forcing. Then if actual experiments can be performed in the upper troposphere, tropopause and the lower stratosphere, the results might be interesting. One reason why is if it's found that co2 and other molecules change then why it's warmer above the ozone layer than below it might get scientists to reconsider how our atmosphere works.
You see, if an app or other software gets updated it's not a problem. With science it seems to not want to update or verify previously accepted assumptions.
Jim
Attached image:
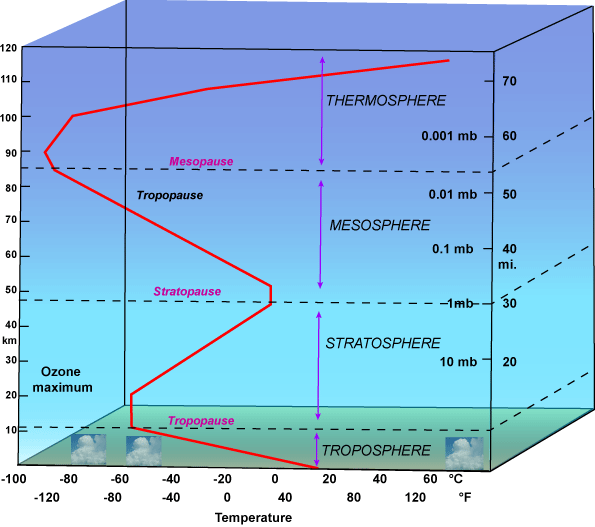 |
| 22-03-2017 23:06 |
Wake★★★★★
(4034) |
James_ wrote:
Wake wrote:
James_ wrote: I think as for myself I will continue to pursue my experiment and question the half life of stratospheric ozone. There is research that suggests that stratospheric ozone has a much longer half life.
One direction of study might be to calculate the actual temperature of ozone molecules in the upper atmosphere. Remember that they are directly heated by UV from the Sun's emissions and hence tend to have, individually a very high temperature. This hastens to half life a great deal and my guess is the half life in the ozone region to be in seconds rather than in hours. But since it is not only destroyed but formed as well it is difficult to measure and must be calculated.
Wake,
One reason for the experiment is to see if there is atmospheric forcing. Then if actual experiments can be performed in the upper troposphere, tropopause and the lower stratosphere, the results might be interesting. One reason why is if it's found that co2 and other molecules change then why it's warmer above the ozone layer than below it might get scientists to reconsider how our atmosphere works.
You see, if an app or other software gets updated it's not a problem. With science it seems to not want to update or verify previously accepted assumptions. Jim
I wish you the very best of luck. I'm afraid that science in general staying out of the AGW fray has greatly hurt the normally weak funding problems.
The AGW people have created such an atmosphere of fear among normal scientists that they would be made a laughing stock that they have backed off where they should have doubled down on the absolutely preposterous claims that comprise the majority of "science" behind AGW.
As for my comments about O3 being individually hot:
http://www.atmos-meas-tech.net/7/609/2014/amt-7-609-2014.pdf
On figure 3 you can see a very large peak that is not mentioned in most texts concerning ozone.
If you look at:
http://articles.adsabs.harvard.edu//full/1963SSRv....2....3T/0000003.000.html
Looking at figure 5 you will see that the Sun happens to have a peak AT THAT POINT as well.
http://nist.gov/data/PDFfiles/jpcrd8.pdf
Shows the spectrum of O2. This is a terribly complicated paper that shows a couple of things - that the O2 in the atmosphere absorbs energy in so many different bands that it isn't funny. The absolute absorption is rather small.
But it appears to be missing that peak in the Sun's emission and O3's absorption.
As far as I can determine, N2 is transparent in the UV region. This would mean that a measurement of temperature would be an average of the temperatures of 99.9% of the gases and O3 could easily be very much hotter than the surrounding atmosphere which as I said could drive the a continuous and quite rapid lifespan of individual ozone molecules at the very time the UV is also constructing new ones. |
| 23-03-2017 13:34 |
Surface Detail★★★★☆
(1673) |
Wake wrote:
This would mean that a measurement of temperature would be an average of the temperatures of 99.9% of the gases and O3 could easily be very much hotter than the surrounding atmosphere which as I said could drive the a continuous and quite rapid lifespan of individual ozone molecules at the very time the UV is also constructing new ones.
As I've already pointed out to you, this is thermodynamically impossible. Two mixed gases at stratospheric pressures cannot have different temperatures because their molecules undergo roughly a million collisions per second.
Please feel free to continue, though. No need to let any actual science get in the way of your fantasies. It's certainly never stopped you before. |
| 23-03-2017 16:02 |
Frescomexico★★☆☆☆
(179) |
Into the Night wrote:
Ozone's biggest threat is ozone. The stuff self destructs to oxygen after a few hours. That process is aided by UV-C sunlight at the top of the stratosphere.
The ozone layer is depleted every night. It is rebuilt every day. As long as you have sunlight and oxygen, you WILL have ozone. We couldn't destroy the ozone layer even if we wanted to.
The CFC ban involved R-12 refrigerant, a substance that Dow Chemical was losing the patent on. They created the 'Ozone Scare' to force everyone onto R-134a, the new substance they just got a patent for.
It was Dow Chemical using the government and the news media to maintain its monopoly.
CFC's are heavier than air. They are like propane. They tend to go down, not up. They are also inert. As a CFC, they will not affect ozone. They must be broken up first.
The component supposedly that DOES affect the ozone layer in CFC's is chlorine. This is a VERY reactive substance. Free chlorine will react with something else before it gets anywhere near the ozone layer. The only 'crisis' was a politically manufactured one.
NOAA basically copies the IPCC graph. They are not capable of producing a graph of global temperatures. No one is. We don't have sufficient instrumentation to produce such a graph. We never have.
I am now a marine refrigeration technician and so have followed the demise of CFCs and now HCFCs rather closely. I can't prove it but I think the ozone hole controversy was just a fortunate coincidence for DuPont, and I can't blame them for not disputing it. It has actually brought me more work and revenue but I still wish it hadn't happened. R-12 and R-22 are superior refrigerants to R-134a and R-410. |
| 23-03-2017 19:53 |
James_★★★★★
(2238) |
@Wake,
>> I wish you the very best of luck. <<
Thank You.
>> I'm afraid that science in general staying out of the AGW fray has greatly hurt the normally weak funding problems. <<
I agree with you on this.
>> As for my comments about O3 being individually hot:
http://www.atmos-meas-tech.net/7/609/2014/amt-7-609-2014.pdf
On figure 3 you can see a very large peak that is not mentioned in most texts concerning ozone.
If you look at:
http://articles.adsabs.harvard.edu//full/1963SSRv....2....3T/0000003.000.html
Looking at figure 5 you will see that the Sun happens to have a peak AT THAT POINT as well.
http://nist.gov/data/PDFfiles/jpcrd8.pdf <<
I think you found something important. This would / could explain how ozone reflects heat back out into space. It's not based on absorption / emission. Basic physics states that the same frequency melds into a greater amplitude frequency. This could be an example of that and why the stratospheric ozone layer is so important.
>> Shows the spectrum of O2. This is a terribly complicated paper that shows a couple of things - that the O2 in the atmosphere absorbs energy in so many different bands that it isn't funny. The absolute absorption is rather small.
But it appears to be missing that peak in the Sun's emission and O3's absorption. <<
It is possible that more O2 type oxygen allows for a warmer planet. And depending on how it influences nitrogen by transferring angular momentum this could increase it's overall effect.
@Surface Detail,
>> Two mixed gases at stratospheric pressures cannot have different temperatures because their molecules undergo roughly a million collisions per second <<
They can. Heat in the atmosphere can be relative to the number of collisions between 2 molecules or gases (nitrogen is an element). This would explain how heat is "trapped" in our atmosphere.
An example of this is the Arctic and Antarctica. This is a neat trick. Neither pole has it's atmosphere attracted to the Van Allen Radiation Belt. Because of this their atmosphere's are not expanded resulting in much fewer collisions.
This would be an example of Maxwell's Demon. https://www.auburn.edu/~smith01/notes/maxdem.htm
And for what we are discussing, the Van Allen Radiation belt would be the Heat Engine. Pretty cool, huh ?
@Frescomexico,
I think there is still much to learn about how our atmosphere works. CFC's have been proven to destroy ozone which is why they were banned in 1987. http://www.sciencemag.org/news/2016/06/ozone-layer-mend-thanks-chemical-ban
Still, there are F-gases as well as other gasses. The link is to the EPA's overview which omits Carbon Tetrahydrochloride https://www.nasa.gov/press/2014/august/ozone-depleting-compound-persists-nasa-research-shows/
It could be that as soon as we solve one problem we find we have created another one and am glad to hear it has helped your business.
Jim |
| RE: Van Allen Radiation Belt23-03-2017 22:12 |
James_★★★★★
(2238) |
@All,
If you look at the attached image of the Van Allen Radiation Belt you will see that it exists only warm areas on our planet.
Between the Tropic of Cancer and the Tropic of Capricorn is the tropics as well as the inner belt of the Van Allen Radiation Belt. Outside of the 2 tropics is the temperate climate on our planet which is effected by the outer belt.
And if anyone looks, the Van Allen Radiation Belt absorbs solar radiation that goes beyond the poles on our planet. And this does effect temperature. That is one reason why the stratosphere above the ozone layer is warmer.
Why this matters is that the tropopause might be a Joule-Thompson Throttling Process. https://en.wikipedia.org/wiki/Joule%E2%80%93Thomson_effect
@Wake, One thing I would like to see tested is ozone in the stratosphere. Ground level ozone destroys plant life because as you pointed out, it is hot. In the ozone layer it might be a little cooler. The link says that 3 gasses do not cool when expanded using the Joules - Thompson Throttling Process.
This allows for the possibility that ozone can absorb solar radiation and then emit it towards the more excited part of our atmosphere, the upper stratosphere. This can help to explain why the upper stratosphere has been cooling as the ozone layer thinned.
http://research.omicsgroup.org/index.php/Joule%E2%80%93Thomson_effect
https://goo.gl/photos/K4Zu3xQXubnC4BX27
Edited on 23-03-2017 22:27 |
| 23-03-2017 23:13 |
Into the Night ★★★★★ ★★★★★
(21598) |
Surface Detail wrote:
Into the Night wrote:
Wake wrote:
spot wrote:
Into the Night wrote:
Ozone's biggest threat is ozone. The stuff self destructs to oxygen after a few hours. That process is aided by UV-C sunlight at the top of the stratosphere.
The ozone layer is depleted every night. It is rebuilt every day. As long as you have sunlight and oxygen, you WILL have ozone. We couldn't destroy the ozone layer even if we wanted to.
The CFC ban involved R-12 refrigerant, a substance that Dow Chemical was losing the patent on. They created the 'Ozone Scare' to force everyone onto R-134a, the new substance they just got a patent for.
It was Dow Chemical using the government and the news media to maintain its monopoly.
CFC's are heavier than air. They are like propane. They tend to go down, not up. They are also inert. As a CFC, they will not affect ozone. They must be broken up first.
The component supposedly that DOES affect the ozone layer in CFC's is chlorine. This is a VERY reactive substance. Free chlorine will react with something else before it gets anywhere near the ozone layer. The only 'crisis' was a politically manufactured one.
NOAA basically copies the IPCC graph. They are not capable of producing a graph of global temperatures. No one is. We don't have sufficient instrumentation to produce such a graph. We never have.
That's a fantastic post, I of course mean fantastic in the sense that it's a product of fantasy.
You simply cannot contain your ignorance can you?
"One of the founding fathers of the French ecological movement and a former minister for the prevention of major natural and technological risks, Tazieff is well qualified to talk about environmental issues. He has been adviser to most of France's environment ministers over the past decade. Despite this he asserts that Green parties are running a "campaign of deliberate, untruthful scaremongering," and the imaginary problems they espouse have led to millions of pounds being directed towards "environmental windmills" rather than the real threats of pollution. It seemed strange to Tazieff that an ozone hole situated above the Antarctic was blamed on CFC gases, when most deodorants were sprayed in the northern hemisphere. He was surprised to discover an article in the 1950 Annals of Geophysics reporting the existence of ozone holes above Norway in 1926 - years before CFC's were even dreamt of - and was astounded to find that the hole above the Antarctic was not the recent phenomenon ecologists claimed it to be. It was actually discovered as far back as 1957, he says, by the English scientist, Gordon Dobson, but it was only in the mid-eighties that satellite photos began to highlight it in a rather spectacular way.
Tazieff believes that these dramatic images have been used to hoodwink the public. He believes that the hole is due to the low levels of ultraviolet rays (which are necessary to produce ozone) over the Antarctic at the end of the year, and that the large and swift movements of air masses around the continent also play their part. On September 5, 1987, there was a relatively large reduction of 0.1 per cent in the levels of ozone over a surface of three million square kilometres near the Palmer peninsula in the Antarctic. Tazieff is convinced there is no way that the CFCs could have broken down so much ozone in such a short space of time."
If you expose ozone to CFC's, nothing happens.
If you break up the CFC's into chlorine and fluorocarbon, the chlorine reacts to destroy ozone. It also reacts with practically everything else. The fluorocarbon component remains stable and doesn't take part in the reaction.
The biggest 'danger' to ozone is ozone itself. It naturally decomposes back to oxygen in a few hours once the energy producing it has been removed. Exposure to UV-C also destroys ozone.
Ozone is created at the bottom of the stratosphere (which is why the tropopause is so cold) by UV-B and UV-A action on oxygen. By this altitude, UV-C has been completely absorbed by the destruction of ozone at the top of the stratosphere (which is why it gets hotter as you rise in the stratosphere).
At night, no Sun, no ozone production or destruction. Decay begins immediately and progresses throughout the night. The ozone layer is rebuilt again when the Sun rises. This delay is why you burn in the late morning (you don't feel that for a few hours after the actual burn, which makes it seem like you burn in the afternoon).
As long as you have sunlight and oxygen, you WILL have ozone.
According to this site:
http://www.lenntech.com/library/ozone/decomposition/ozone-decomposition.htm
the half-life of ozone in air at a temperature of -50°C (the approximate temperature of the ozone layer) is 3 months, not a few hours.
This actual fact rather scuppers your argument, does it not?
Not at all. The temperature of the lower part of the stratosphere, where ozone is produced, can be as low as-80degC. The top, is just below freezing, around -5degC. You also failed to account for the pressure change and the additional UV-C light I already mentioned.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 23-03-2017 23:22 |
Into the Night ★★★★★ ★★★★★
(21598) |
Surface Detail wrote:
Wake wrote:
James_ wrote: I think as for myself I will continue to pursue my experiment and question the half life of stratospheric ozone. There is research that suggests that stratospheric ozone has a much longer half life.
One direction of study might be to calculate the actual temperature of ozone molecules in the upper atmosphere. Remember that they are directly heated by UV from the Sun's emissions and hence tend to have, individually a very high temperature. This hastens to half life a great deal and my guess is the half life in the ozone region to be in seconds rather than in hours. But since it is not only destroyed but formed as well it is difficult to measure and must be calculated.
Wrong.
The pressure and temperature in the ozone layer are about 50 mbar and -50C respectively. Plugging those figures together with a mean molecular diameter of 2.5 nm and mass of 29 amu for air into the site below gives a collision frequency of about 2 x 10^6 per second. This means that the ozone molecules reach thermal equilibrium with the surrounding molecules within a few microseconds. At -50C, the half-life of the ozone will be about 3 months.
http://hyperphysics.phy-astr.gsu.edu/hbase/Kinetic/frecol.html#c1
Ozone is a gas...you know...it moves around. It doesn't spend all day in the place it was formed. It is destroyed by itself, and UV-C.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 23-03-2017 23:31 |
Wake★★★★★
(4034) |
James_ wrote:
@Wake, One thing I would like to see tested is ozone in the stratosphere. Ground level ozone destroys plant life because as you pointed out, it is hot. In the ozone layer it might be a little cooler. The link says that 3 gasses do not cool when expanded using the Joules - Thompson Throttling Process.
This allows for the possibility that ozone can absorb solar radiation and then emit it towards the more excited part of our atmosphere, the upper stratosphere. This can help to explain why the upper stratosphere has been cooling as the ozone layer thinned.
http://research.omicsgroup.org/index.php/Joule%E2%80%93Thomson_effect
https://goo.gl/photos/K4Zu3xQXubnC4BX27
Remember that radiation is a 360 degree occurrence. So as much energy is lost back to space as is radiated in a downward direction. Unlike Surface defect seems to believe, in the stratosphere the molecules are too far apart to transmit much heat via conduction. He seems to believe that in a thin atmosphere that the molecules race around like cars on a track. |
| 24-03-2017 03:18 |
Surface Detail★★★★☆
(1673) |
Into the Night wrote:
Surface Detail wrote:
Into the Night wrote:
Wake wrote:
spot wrote:
Into the Night wrote:
Ozone's biggest threat is ozone. The stuff self destructs to oxygen after a few hours. That process is aided by UV-C sunlight at the top of the stratosphere.
The ozone layer is depleted every night. It is rebuilt every day. As long as you have sunlight and oxygen, you WILL have ozone. We couldn't destroy the ozone layer even if we wanted to.
The CFC ban involved R-12 refrigerant, a substance that Dow Chemical was losing the patent on. They created the 'Ozone Scare' to force everyone onto R-134a, the new substance they just got a patent for.
It was Dow Chemical using the government and the news media to maintain its monopoly.
CFC's are heavier than air. They are like propane. They tend to go down, not up. They are also inert. As a CFC, they will not affect ozone. They must be broken up first.
The component supposedly that DOES affect the ozone layer in CFC's is chlorine. This is a VERY reactive substance. Free chlorine will react with something else before it gets anywhere near the ozone layer. The only 'crisis' was a politically manufactured one.
NOAA basically copies the IPCC graph. They are not capable of producing a graph of global temperatures. No one is. We don't have sufficient instrumentation to produce such a graph. We never have.
That's a fantastic post, I of course mean fantastic in the sense that it's a product of fantasy.
You simply cannot contain your ignorance can you?
"One of the founding fathers of the French ecological movement and a former minister for the prevention of major natural and technological risks, Tazieff is well qualified to talk about environmental issues. He has been adviser to most of France's environment ministers over the past decade. Despite this he asserts that Green parties are running a "campaign of deliberate, untruthful scaremongering," and the imaginary problems they espouse have led to millions of pounds being directed towards "environmental windmills" rather than the real threats of pollution. It seemed strange to Tazieff that an ozone hole situated above the Antarctic was blamed on CFC gases, when most deodorants were sprayed in the northern hemisphere. He was surprised to discover an article in the 1950 Annals of Geophysics reporting the existence of ozone holes above Norway in 1926 - years before CFC's were even dreamt of - and was astounded to find that the hole above the Antarctic was not the recent phenomenon ecologists claimed it to be. It was actually discovered as far back as 1957, he says, by the English scientist, Gordon Dobson, but it was only in the mid-eighties that satellite photos began to highlight it in a rather spectacular way.
Tazieff believes that these dramatic images have been used to hoodwink the public. He believes that the hole is due to the low levels of ultraviolet rays (which are necessary to produce ozone) over the Antarctic at the end of the year, and that the large and swift movements of air masses around the continent also play their part. On September 5, 1987, there was a relatively large reduction of 0.1 per cent in the levels of ozone over a surface of three million square kilometres near the Palmer peninsula in the Antarctic. Tazieff is convinced there is no way that the CFCs could have broken down so much ozone in such a short space of time."
If you expose ozone to CFC's, nothing happens.
If you break up the CFC's into chlorine and fluorocarbon, the chlorine reacts to destroy ozone. It also reacts with practically everything else. The fluorocarbon component remains stable and doesn't take part in the reaction.
The biggest 'danger' to ozone is ozone itself. It naturally decomposes back to oxygen in a few hours once the energy producing it has been removed. Exposure to UV-C also destroys ozone.
Ozone is created at the bottom of the stratosphere (which is why the tropopause is so cold) by UV-B and UV-A action on oxygen. By this altitude, UV-C has been completely absorbed by the destruction of ozone at the top of the stratosphere (which is why it gets hotter as you rise in the stratosphere).
At night, no Sun, no ozone production or destruction. Decay begins immediately and progresses throughout the night. The ozone layer is rebuilt again when the Sun rises. This delay is why you burn in the late morning (you don't feel that for a few hours after the actual burn, which makes it seem like you burn in the afternoon).
As long as you have sunlight and oxygen, you WILL have ozone.
According to this site:
http://www.lenntech.com/library/ozone/decomposition/ozone-decomposition.htm
the half-life of ozone in air at a temperature of -50°C (the approximate temperature of the ozone layer) is 3 months, not a few hours.
This actual fact rather scuppers your argument, does it not?
Not at all. The temperature of the lower part of the stratosphere, where ozone is produced, can be as low as-80degC. The top, is just below freezing, around -5degC. You also failed to account for the pressure change and the additional UV-C light I already mentioned.
The pressure makes no difference to the half-life, and there is no UV-C during the night. The ozone layer does not vanish during the night. |
| 24-03-2017 15:56 |
Wake★★★★★
(4034) |
Surface Detail wrote:
The pressure makes no difference to the half-life, and there is no UV-C during the night. The ozone layer does not vanish during the night.
A real atmospheric chemist I see. Perhaps you should learn something before spouting it. |
| 24-03-2017 16:35 |
James_★★★★★
(2238) |
Surface Detail,
It could matter how they cool the field that ozone is in. If they are cooling it via conduction then that is very different than a Joule - Thomson Throttling Process. And with me I would say that ozone hasn't been cooled in this manner. One reason why is that ozone wouldn't stay in that field. They would need to maintain a Joule - Thomson Field with no airflow to observe ozone in a field similar to the stratosphere. And if they did that then they could under controlled conditions study ozone better.
This is one reason I've been wanting to see my experiment tried. If successful then it would show that the upper atmosphere works differently than is currently believed.
Jim |
|
| 24-03-2017 19:37 |
Into the Night ★★★★★ ★★★★★
(21598) |
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
Into the Night wrote:
Wake wrote:
spot wrote:
Into the Night wrote:
Ozone's biggest threat is ozone. The stuff self destructs to oxygen after a few hours. That process is aided by UV-C sunlight at the top of the stratosphere.
The ozone layer is depleted every night. It is rebuilt every day. As long as you have sunlight and oxygen, you WILL have ozone. We couldn't destroy the ozone layer even if we wanted to.
The CFC ban involved R-12 refrigerant, a substance that Dow Chemical was losing the patent on. They created the 'Ozone Scare' to force everyone onto R-134a, the new substance they just got a patent for.
It was Dow Chemical using the government and the news media to maintain its monopoly.
CFC's are heavier than air. They are like propane. They tend to go down, not up. They are also inert. As a CFC, they will not affect ozone. They must be broken up first.
The component supposedly that DOES affect the ozone layer in CFC's is chlorine. This is a VERY reactive substance. Free chlorine will react with something else before it gets anywhere near the ozone layer. The only 'crisis' was a politically manufactured one.
NOAA basically copies the IPCC graph. They are not capable of producing a graph of global temperatures. No one is. We don't have sufficient instrumentation to produce such a graph. We never have.
That's a fantastic post, I of course mean fantastic in the sense that it's a product of fantasy.
You simply cannot contain your ignorance can you?
"One of the founding fathers of the French ecological movement and a former minister for the prevention of major natural and technological risks, Tazieff is well qualified to talk about environmental issues. He has been adviser to most of France's environment ministers over the past decade. Despite this he asserts that Green parties are running a "campaign of deliberate, untruthful scaremongering," and the imaginary problems they espouse have led to millions of pounds being directed towards "environmental windmills" rather than the real threats of pollution. It seemed strange to Tazieff that an ozone hole situated above the Antarctic was blamed on CFC gases, when most deodorants were sprayed in the northern hemisphere. He was surprised to discover an article in the 1950 Annals of Geophysics reporting the existence of ozone holes above Norway in 1926 - years before CFC's were even dreamt of - and was astounded to find that the hole above the Antarctic was not the recent phenomenon ecologists claimed it to be. It was actually discovered as far back as 1957, he says, by the English scientist, Gordon Dobson, but it was only in the mid-eighties that satellite photos began to highlight it in a rather spectacular way.
Tazieff believes that these dramatic images have been used to hoodwink the public. He believes that the hole is due to the low levels of ultraviolet rays (which are necessary to produce ozone) over the Antarctic at the end of the year, and that the large and swift movements of air masses around the continent also play their part. On September 5, 1987, there was a relatively large reduction of 0.1 per cent in the levels of ozone over a surface of three million square kilometres near the Palmer peninsula in the Antarctic. Tazieff is convinced there is no way that the CFCs could have broken down so much ozone in such a short space of time."
If you expose ozone to CFC's, nothing happens.
If you break up the CFC's into chlorine and fluorocarbon, the chlorine reacts to destroy ozone. It also reacts with practically everything else. The fluorocarbon component remains stable and doesn't take part in the reaction.
The biggest 'danger' to ozone is ozone itself. It naturally decomposes back to oxygen in a few hours once the energy producing it has been removed. Exposure to UV-C also destroys ozone.
Ozone is created at the bottom of the stratosphere (which is why the tropopause is so cold) by UV-B and UV-A action on oxygen. By this altitude, UV-C has been completely absorbed by the destruction of ozone at the top of the stratosphere (which is why it gets hotter as you rise in the stratosphere).
At night, no Sun, no ozone production or destruction. Decay begins immediately and progresses throughout the night. The ozone layer is rebuilt again when the Sun rises. This delay is why you burn in the late morning (you don't feel that for a few hours after the actual burn, which makes it seem like you burn in the afternoon).
As long as you have sunlight and oxygen, you WILL have ozone.
According to this site:
http://www.lenntech.com/library/ozone/decomposition/ozone-decomposition.htm
the half-life of ozone in air at a temperature of -50°C (the approximate temperature of the ozone layer) is 3 months, not a few hours.
This actual fact rather scuppers your argument, does it not?
Not at all. The temperature of the lower part of the stratosphere, where ozone is produced, can be as low as-80degC. The top, is just below freezing, around -5degC. You also failed to account for the pressure change and the additional UV-C light I already mentioned.
The pressure makes no difference to the half-life, and there is no UV-C during the night. The ozone layer does not vanish during the night.
Yes it does. Any radioman know that and USES that fact in his work.
As soon as the Sun goes down, ozone depletion begins. During the longer winter nights, ozone depletion is almost complete, except for a thin layer at the top called the F layer.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 24-03-2017 19:49 |
spot★★★★☆
(1323) |
Into the Night wrote:
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
Into the Night wrote:
Wake wrote:
spot wrote:
Into the Night wrote:
Ozone's biggest threat is ozone. The stuff self destructs to oxygen after a few hours. That process is aided by UV-C sunlight at the top of the stratosphere.
The ozone layer is depleted every night. It is rebuilt every day. As long as you have sunlight and oxygen, you WILL have ozone. We couldn't destroy the ozone layer even if we wanted to.
The CFC ban involved R-12 refrigerant, a substance that Dow Chemical was losing the patent on. They created the 'Ozone Scare' to force everyone onto R-134a, the new substance they just got a patent for.
It was Dow Chemical using the government and the news media to maintain its monopoly.
CFC's are heavier than air. They are like propane. They tend to go down, not up. They are also inert. As a CFC, they will not affect ozone. They must be broken up first.
The component supposedly that DOES affect the ozone layer in CFC's is chlorine. This is a VERY reactive substance. Free chlorine will react with something else before it gets anywhere near the ozone layer. The only 'crisis' was a politically manufactured one.
NOAA basically copies the IPCC graph. They are not capable of producing a graph of global temperatures. No one is. We don't have sufficient instrumentation to produce such a graph. We never have.
That's a fantastic post, I of course mean fantastic in the sense that it's a product of fantasy.
You simply cannot contain your ignorance can you?
"One of the founding fathers of the French ecological movement and a former minister for the prevention of major natural and technological risks, Tazieff is well qualified to talk about environmental issues. He has been adviser to most of France's environment ministers over the past decade. Despite this he asserts that Green parties are running a "campaign of deliberate, untruthful scaremongering," and the imaginary problems they espouse have led to millions of pounds being directed towards "environmental windmills" rather than the real threats of pollution. It seemed strange to Tazieff that an ozone hole situated above the Antarctic was blamed on CFC gases, when most deodorants were sprayed in the northern hemisphere. He was surprised to discover an article in the 1950 Annals of Geophysics reporting the existence of ozone holes above Norway in 1926 - years before CFC's were even dreamt of - and was astounded to find that the hole above the Antarctic was not the recent phenomenon ecologists claimed it to be. It was actually discovered as far back as 1957, he says, by the English scientist, Gordon Dobson, but it was only in the mid-eighties that satellite photos began to highlight it in a rather spectacular way.
Tazieff believes that these dramatic images have been used to hoodwink the public. He believes that the hole is due to the low levels of ultraviolet rays (which are necessary to produce ozone) over the Antarctic at the end of the year, and that the large and swift movements of air masses around the continent also play their part. On September 5, 1987, there was a relatively large reduction of 0.1 per cent in the levels of ozone over a surface of three million square kilometres near the Palmer peninsula in the Antarctic. Tazieff is convinced there is no way that the CFCs could have broken down so much ozone in such a short space of time."
If you expose ozone to CFC's, nothing happens.
If you break up the CFC's into chlorine and fluorocarbon, the chlorine reacts to destroy ozone. It also reacts with practically everything else. The fluorocarbon component remains stable and doesn't take part in the reaction.
The biggest 'danger' to ozone is ozone itself. It naturally decomposes back to oxygen in a few hours once the energy producing it has been removed. Exposure to UV-C also destroys ozone.
Ozone is created at the bottom of the stratosphere (which is why the tropopause is so cold) by UV-B and UV-A action on oxygen. By this altitude, UV-C has been completely absorbed by the destruction of ozone at the top of the stratosphere (which is why it gets hotter as you rise in the stratosphere).
At night, no Sun, no ozone production or destruction. Decay begins immediately and progresses throughout the night. The ozone layer is rebuilt again when the Sun rises. This delay is why you burn in the late morning (you don't feel that for a few hours after the actual burn, which makes it seem like you burn in the afternoon).
As long as you have sunlight and oxygen, you WILL have ozone.
According to this site:
http://www.lenntech.com/library/ozone/decomposition/ozone-decomposition.htm
the half-life of ozone in air at a temperature of -50°C (the approximate temperature of the ozone layer) is 3 months, not a few hours.
This actual fact rather scuppers your argument, does it not?
Not at all. The temperature of the lower part of the stratosphere, where ozone is produced, can be as low as-80degC. The top, is just below freezing, around -5degC. You also failed to account for the pressure change and the additional UV-C light I already mentioned.
The pressure makes no difference to the half-life, and there is no UV-C during the night. The ozone layer does not vanish during the night.
Yes it does. Any radioman know that and USES that fact in his work.
As soon as the Sun goes down, ozone depletion begins. During the longer winter nights, ozone depletion is almost complete, except for a thin layer at the top called the F layer.
Fcuk me you are a radioman now.
I don't think radio waves work the way you think they work, muppet.
IBdaMann wrote:
"Air" is not a body in and of itself. Ergo it is not a blackbody.
Planck's law describes the spectral density of electromagnetic radiation emitted by a black body in thermal equilibrium at a given temperature T.
Edited on 24-03-2017 19:51 |
| 24-03-2017 22:04 |
Surface Detail★★★★☆
(1673) |
Into the Night wrote:
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
According to this site:
http://www.lenntech.com/library/ozone/decomposition/ozone-decomposition.htm
the half-life of ozone in air at a temperature of -50°C (the approximate temperature of the ozone layer) is 3 months, not a few hours.
This actual fact rather scuppers your argument, does it not?
Not at all. The temperature of the lower part of the stratosphere, where ozone is produced, can be as low as-80degC. The top, is just below freezing, around -5degC. You also failed to account for the pressure change and the additional UV-C light I already mentioned.
The pressure makes no difference to the half-life, and there is no UV-C during the night. The ozone layer does not vanish during the night.
Yes it does. Any radioman know that and USES that fact in his work.
As soon as the Sun goes down, ozone depletion begins. During the longer winter nights, ozone depletion is almost complete, except for a thin layer at the top called the F layer.
You seem to have confused the ozone layer with the ionosphere. A real radioman would know that radio waves are reflected by the ionosphere (parts of which do indeed disappear at night), not the ozone layer (which doesn't disappear at night). |
| 24-03-2017 23:21 |
Wake★★★★★
(4034) |
Surface Detail wrote:
YiuYou seem to have confused the ozone layer with the ionosphere. A real radioman would know that radio waves are reflected by the ionosphere (parts of which do indeed disappear at night), not the ozone layer (which doesn't disappear at night).
You seem to have the idea that someone give's a shit. I will say that the last time I met a moron like you he was using a walker and couldn't look straight forward. |
| 25-03-2017 00:04 |
spot★★★★☆
(1323) |
Wake wrote:
Surface Detail wrote:
YiuYou seem to have confused the ozone layer with the ionosphere. A real radioman would know that radio waves are reflected by the ionosphere (parts of which do indeed disappear at night), not the ozone layer (which doesn't disappear at night).
You seem to have the idea that someone give's a shit. I will say that the last time I met a moron like you he was using a walker and couldn't look straight forward.
You don't give a shit about what is true and what is false. You're just posting here because you have been banned from everywhere else
That much we do understand.
IBdaMann wrote:
"Air" is not a body in and of itself. Ergo it is not a blackbody.
Planck's law describes the spectral density of electromagnetic radiation emitted by a black body in thermal equilibrium at a given temperature T. |
| 25-03-2017 00:10 |
Surface Detail★★★★☆
(1673) |
Wake wrote:
Surface Detail wrote:
YiuYou seem to have confused the ozone layer with the ionosphere. A real radioman would know that radio waves are reflected by the ionosphere (parts of which do indeed disappear at night), not the ozone layer (which doesn't disappear at night).
You seem to have the idea that someone give's a shit. I will say that the last time I met a moron like you he was using a walker and couldn't look straight forward.
When your opponent gives up arguing completely and descends into pure abuse, you know you've won the argument. Well done me |
| 25-03-2017 00:14 |
spot★★★★☆
(1323) |
Surface Detail wrote:
Wake wrote:
Surface Detail wrote:
YiuYou seem to have confused the ozone layer with the ionosphere. A real radioman would know that radio waves are reflected by the ionosphere (parts of which do indeed disappear at night), not the ozone layer (which doesn't disappear at night).
You seem to have the idea that someone give's a shit. I will say that the last time I met a moron like you he was using a walker and couldn't look straight forward.
When your opponent gives up arguing completely and descends into pure abuse, you know you've won the argument. Well done me
Congratulations!!!!

And its got Al Gore in the image.
Sorry, it's not constructive and will probably rile him into an all night posting session but I could not resist.
IBdaMann wrote:
"Air" is not a body in and of itself. Ergo it is not a blackbody.
Planck's law describes the spectral density of electromagnetic radiation emitted by a black body in thermal equilibrium at a given temperature T. |
| 25-03-2017 00:42 |
Wake★★★★★
(4034) |
spot wrote:
Wake wrote:
Surface Detail wrote:
YiuYou seem to have confused the ozone layer with the ionosphere. A real radioman would know that radio waves are reflected by the ionosphere (parts of which do indeed disappear at night), not the ozone layer (which doesn't disappear at night).
You seem to have the idea that someone give's a shit. I will say that the last time I met a moron like you he was using a walker and couldn't look straight forward.
You don't give a shit about what is true and what is false. You're just posting here because you have been banned from everywhere else
That much we do understand.
I don't see you on any other board because you can't even hold a candle to the half-scientists that post there. |
| 27-03-2017 00:12 |
Into the Night ★★★★★ ★★★★★
(21598) |
spot wrote:
Into the Night wrote:
Yes it does. Any radioman know that and USES that fact in his work.
As soon as the Sun goes down, ozone depletion begins. During the longer winter nights, ozone depletion is almost complete, except for a thin layer at the top called the F layer.
Fcuk me you are a radioman now.
I don't think radio waves work the way you think they work, muppet.
Yes...I am a radioman...a pilot...an aircraft builder and designer...an instrumentation engineer...a musician...an aircraft and car mechanic...a home owner...an electrical and electronics engineer...a scientist...a welder...a software engineer...a father...and a whole host of other things.
You have a problem with that...obviously.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 27-03-2017 00:14 |
Into the Night ★★★★★ ★★★★★
(21598) |
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
Into the Night wrote:
Surface Detail wrote:
According to this site:
http://www.lenntech.com/library/ozone/decomposition/ozone-decomposition.htm
the half-life of ozone in air at a temperature of -50°C (the approximate temperature of the ozone layer) is 3 months, not a few hours.
This actual fact rather scuppers your argument, does it not?
Not at all. The temperature of the lower part of the stratosphere, where ozone is produced, can be as low as-80degC. The top, is just below freezing, around -5degC. You also failed to account for the pressure change and the additional UV-C light I already mentioned.
The pressure makes no difference to the half-life, and there is no UV-C during the night. The ozone layer does not vanish during the night.
Yes it does. Any radioman know that and USES that fact in his work.
As soon as the Sun goes down, ozone depletion begins. During the longer winter nights, ozone depletion is almost complete, except for a thin layer at the top called the F layer.
You seem to have confused the ozone layer with the ionosphere. A real radioman would know that radio waves are reflected by the ionosphere (parts of which do indeed disappear at night), not the ozone layer (which doesn't disappear at night).
Radio waves are reflected by BOTH. The reflection is dependent on the frequency used.
A narrow band of frequencies are not reflected by EITHER. These are the so-called 'space' frequencies. They are the only frequencies available to communicate with satellites and other spacecraft.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |

