| polar vortex18-02-2021 03:40 |
duncan61★★★★★
(2021) |
I had a spare hour this morning so I went looking for articles on how warming is making it cold and found 3 articles instantly less than 3 hours old.Apparently because we have warmed the Arctic with CO2 it has released the polar vortex and let the gulf stream out.Then a unicorn turned up and made it all better
duncan61 |
| 18-02-2021 04:36 |
IBdaMann ★★★★★ ★★★★★
(14389) |
duncan61 wrote: I had a spare hour this morning so I went looking for articles on how warming is making it cold and found 3 articles instantly less than 3 hours old.Apparently because we have warmed the Arctic with CO2 it has released the polar vortex and let the gulf stream out.Then a unicorn turned up and made it all better
Duncan, you are going to give Into the Night and I deja vu if you carry on as such with this topic. We spent over a year with litesong preaching his twice-daily updates of just how blazing hot human activity was cooking the Arctic, causing the Arctic atmosphere to explosively expand, blasting freezing Arctic air southward ... because Global Warming predicted decreasing temperatures.
He referred to the process as Arctic Berserker melting.
25 November 2016 litesong wrote:
Meanwhile:
1990's decade of Arctic sea ice extent to date was 4% less than that of the 1980's.
2000's decade of Arctic sea ice extent to date was 10% less than that of the 1980's.
The year 2012 of Arctic sea ice extent to date was 20% less than that of the 1980's.
The year 2016 of Arctic sea ice extent to date is 23% less than that of the 1980's( 24+% due to the present High Arctic Berserker MELTING sea ice in the time of normal vast INCREASE of sea ice).
The 1980's average November 1 Arctic sea ice VOLUME was 18,100 cubic kilometers. November 1, 2016 Arctic sea ice VOLUME was 6400 cubic kilometers, a loss of 11,700 cubic kilometers or a loss of 64.6%. The energy needed to melt this ice is 36 times the U.S. annual energy consumption. Since 1958, High Arctic temperature has been rising (more before satellite temperatures?). Two plus years ago, High Arctic temperature over nearly 4 million square kilometers, for 140 straight days was over average. For a few days at the end of 2015, the North Pole was THAWING! From the end of 2015 into 2016, there were 150 straight days over average temperature. For 100 straight days during that period, temperatures were 3degC to 11degC over average. For 75+ straight days presently, High Arctic temperatures are & have been over average AND MOSTLY WAY OVER AVERAGE. High Arctic temperatures over nearly 4 million square kilometers presently are 11degC over average, dropping from 20 degC over average. For 10 years the sun has been at a low TSI (including a 3+ year period, setting a 100 year record low). But, Earth temperatures have not returned to early 20th century levels. For 385+(?) straight months, Earth temperatures have been over the 20th century average.
litesong on 2. December 2016, 00:12
The sun has been at low solar TSI for 10 years(including a 3+ year period setting a 100 year record low). But Earth temperatures have not retreated to early 20th century levels. For 385+ straight months, Earth temperatures have been over the 20th century average. Even with the sun at a low solar TSI, Arctic sea ice will disappear ('cept fer a bit? of thickness that likes to adhere to northern Canadian Archipelago & northern Greenland?). If solar TSI returns to normal(higher?), the Arctic sea ice may disappear sooner.
On 16 November 2016 litesong wrote:
With the High Arctic in darkness for almost 2 months to date:
1990's decade of Arctic sea ice extent to date was 4% less than that of the 1980's.
2000's decade of Arctic sea ice extent to date was 10% less than that of the 1980's.
The year 2012 of Arctic sea ice extent to date was 20% less than that of the 1980's.
The year 2016 of Arctic sea ice extent to date is 23% less than that of the 1980's.
The 1980's average November 1 Arctic sea ice VOLUME was 18,100 cubic kilometers. November 1, 2016 Arctic sea ice VOLUME was 6400 cubic kilometers, a loss of 11,700 cubic kilometers or a loss of 64.6%. The energy needed to melt this ice is 36 times the U.S. annual energy consumption. Since 1958, High Arctic temperature has been rising (more before satellite temperatures?). Two plus years ago, High Arctic temperature over nearly 4 million square kilometers, for 140 straight days was over average. For a few days at the end of 2015, the North Pole was THAWING! From the end of 2015 into 2016, there were 150 straight days over average temperature. For 100 straight days during that period, temperatures were 3degC to 11degC over average. For 65 straight days presently, High Arctic temperatures are & have been over average AND MOSTLY WAY OVER AVERAGE. High Arctic temperatures over nearly 4 million square kilometers presently are 18degC over average...I believe the highest anomaly since 1958. For 10 years the sun has been at a low TSI (including a 3+ year period, setting a 100 year record low). But, Earth temperatures have not returned to early 20th century levels. For 385+(?) straight months, Earth temperatures have been over the 20th century average.
Note: litesong was one of the two people banned from this forum. The days of Berserker melting updates are a thing of the past.
.
I don't think i can [define it]. I just kind of get a feel for the phrase. - keepit
A Spaghetti strainer with the faucet running, retains water- tmiddles
Clouds don't trap heat. Clouds block cold. - Spongy Iris
Printing dollars to pay debt doesn't increase the number of dollars. - keepit
If Venus were a black body it would have a much much lower temperature than what we found there.- tmiddles
Ah the "Valid Data" myth of ITN/IBD. - tmiddles
Ceist - I couldn't agree with you more. But when money and religion are involved, and there are people who value them above all else, then the lies begin. - trafn
You are completely misunderstanding their use of the word "accumulation"! - Climate Scientist.
The Stefan-Boltzman equation doesn't come up with the correct temperature if greenhouse gases are not considered - Hank
:*sigh* Not the "raw data" crap. - Leafsdude
IB STILL hasn't explained what Planck's Law means. Just more hand waving that it applies to everything and more asserting that the greenhouse effect 'violates' it.- Ceist |
| 18-02-2021 10:08 |
Spongy Iris ★★★★☆ ★★★★☆
(1643) |
duncan61 wrote:
I had a spare hour this morning so I went looking for articles on how warming is making it cold and found 3 articles instantly less than 3 hours old.Apparently because we have warmed the Arctic with CO2 it has released the polar vortex and let the gulf stream out.Then a unicorn turned up and made it all better
I think the main cause of Arctic air travelling further south in many January and February months is "Sudden Stratospheric Warming" over the north pole. The hot air pushes the cold air further south.
It doesn't appear SSW is well understood, but it is known to happen about 6 times in 10 years.
I have shared this image from NASA many times here of an SSW event that happened in Feb 1989.
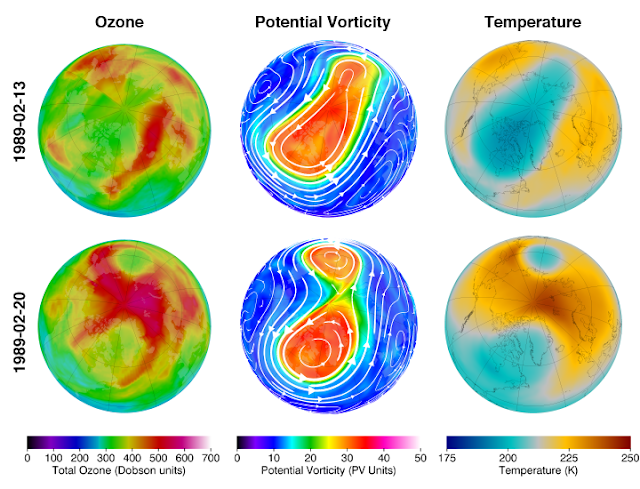
It appears SSW happens the same time that there is an ozone build up over the North Pole.
%20(1).png) |
| 18-02-2021 11:54 |
duncan61★★★★★
(2021) |
I am interested in this Sudden Stratospheric Warming.Question Hot air rises how does it push cold air South.There is no sunlight at the North pole so no Ozone either.How does all this work and who claims to know how it works.I have seen many scientist declare its to complicated for us to know and that makes sense. |
| 18-02-2021 16:05 |
James___★★★★★
(5513) |
The link is to 2 posts (one follows the other) that I made about this. https://www.climate-debate.com/forum/are-we-on-the-brink-of-a-new-little-ice-age-woods-hole-oceanographic-d6-e3538-s80.php#post_69466
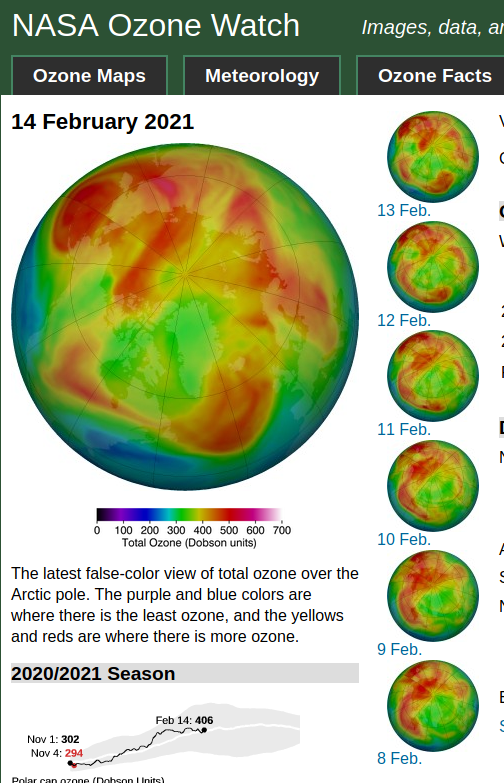
If it was the polar vortex shifting, they would've said that before this happened. It actually seems that the north polar region has expanded. If so, then it would be because of how much ozone there is in the arctic and around the arctic.
I've been saying for a long time that ozone reduces the amount of heat that the stratosphere allows into the troposphere. This tends to support that hypothesis.
The red area to the bottom left is covering the US and the red area to the lower right is over the ocean and borders itself on England. This means cooler air will be blowing over England. And those red areas represents a lot of ozone.
To bring thermodynamics into this. The ozone can circulate from the lower latitudes of the area up into the higher latitudes. Warm to cold. And thus the ozone shield would be maintained. Also, the stratosphere is about 10 miles of altitude. That could keep a good portion of it over the north pole exposed to solar radiation.
What they need to do one day is to place 3 solar panels on top of weather balloons. Then at different altitudes the w/m^2 of solar radiation can be measured. And when the amount of ozone is considered, it would show weather (a pun) or not if the ozone layer helps to cool the troposphere.
Attached image:
Edited on 18-02-2021 16:38 |
|
| 18-02-2021 23:30 |
Spongy Iris ★★★★☆ ★★★★☆
(1643) |
duncan61 wrote:
I am interested in this Sudden Stratospheric Warming.Question Hot air rises how does it push cold air South.There is no sunlight at the North pole so no Ozone either.How does all this work and who claims to know how it works.I have seen many scientist declare its to complicated for us to know and that makes sense.
I cannot seem to find an explanation of the cause of Sudden Stratospheric Warming that makes sense.
But in the image I posted, you should at least be able to read the color changes at the north pole stratosphere, and confirm, one week there's barely any ozone and the temp is approx. 200 K, and next week there's a heavy concentration of ozone, and temp is approx. 250 K.
There must be a process in the stratosphere, which occurs in regular intervals at the North pole, in Jan Feb months, in 6 of every 10 years, when an extra ordinary blast of ozone is produced and cycled into the atmosphere, which generates extra ordinary heat in the North Pole stratosphere, which pushes down on the typical polar jet stream winds.
The process does not appear to involve Sun rays, because it occurs before the North Pole faces the Sun.
%20(1).png) |
| 19-02-2021 01:24 |
IBdaMann ★★★★★ ★★★★★
(14389) |
James___ wrote: To bring thermodynamics into this. The ozone can circulate from the lower latitudes of the area up into the higher latitudes. Warm to cold.
James__, you know I hate to ever douse any idea with even a small tanker truck of ice water ... but you are not discussing thermodynamics ... you are discussing fluid dynamics, i.e. the flow of wind.
James___ wrote: That could keep a good portion of it over the north pole exposed to solar radiation.
That's not how it works. Nothing keeps the ozone exposed to solar radiation; the solar radiation creates the ozone.
James___ wrote: What they need to do one day is to place 3 solar panels on top of weather balloons. Then at different altitudes the w/m^2 of solar radiation can be measured. And when the amount of ozone is considered, it would show weather (a pun) or not if the ozone layer helps to cool the troposphere.
No, it would only show the strength of the measured wavelengths of the solar radiation as well as those same wavelengths from errant sources that reach the solar panel at the proscribed elevations.
Any conclusions you make beyond that will be invalid.
. |
| 19-02-2021 01:43 |
HarveyH55 ★★★★★ ★★★★★
(5196) |
Climatologist measure CO2 concentrations, mostly on the side of active, super-volcano... Where are the Ozone readings. What's the global, average, concentration of Ozone? Do ice core samples, tell us what normal levels were like, hundreds of thousand of years ago, like CO2. Or is it mostly missing, because of the huge hole, over the ice fields? Of, that right, cavemen didn't have spray cans of paint to do their graffiti, and destroy the ozone layer. Correlation, doesn't imply causation... But, it's been the basis for many a profitable scams. |
| 19-02-2021 01:54 |
IBdaMann ★★★★★ ★★★★★
(14389) |
Spongy Iris wrote: There must be a process in the stratosphere, which occurs in regular intervals at the North pole, in Jan Feb months, in 6 of every 10 years, when an extra ordinary blast of ozone is produced and cycled into the atmosphere, which generates extra ordinary heat in the North Pole stratosphere, which pushes down on the typical polar jet stream winds.
Would you mind explaining why there must be such a process?
Spongy Iris wrote: The process does not appear to involve Sun rays, because it occurs before the North Pole faces the Sun.
This promises to be good. Please proceed with the explanation.
.
I don't think i can [define it]. I just kind of get a feel for the phrase. - keepit
A Spaghetti strainer with the faucet running, retains water- tmiddles
Clouds don't trap heat. Clouds block cold. - Spongy Iris
Printing dollars to pay debt doesn't increase the number of dollars. - keepit
If Venus were a black body it would have a much much lower temperature than what we found there.- tmiddles
Ah the "Valid Data" myth of ITN/IBD. - tmiddles
Ceist - I couldn't agree with you more. But when money and religion are involved, and there are people who value them above all else, then the lies begin. - trafn
You are completely misunderstanding their use of the word "accumulation"! - Climate Scientist.
The Stefan-Boltzman equation doesn't come up with the correct temperature if greenhouse gases are not considered - Hank
:*sigh* Not the "raw data" crap. - Leafsdude
IB STILL hasn't explained what Planck's Law means. Just more hand waving that it applies to everything and more asserting that the greenhouse effect 'violates' it.- Ceist |
| 19-02-2021 02:07 |
Spongy Iris ★★★★☆ ★★★★☆
(1643) |
IBdaMann wrote:
Spongy Iris wrote: There must be a process in the stratosphere, which occurs in regular intervals at the North pole, in Jan Feb months, in 6 of every 10 years, when an extra ordinary blast of ozone is produced and cycled into the atmosphere, which generates extra ordinary heat in the North Pole stratosphere, which pushes down on the typical polar jet stream winds.
Would you mind explaining why there must be such a process?
Spongy Iris wrote: The process does not appear to involve Sun rays, because it occurs before the North Pole faces the Sun.
This promises to be good. Please proceed with the explanation.
.
Because the stratosphere suddenly warms! Come on man you're going in circles!
Are you asking me to speculate what the process is??
%20(1).png) |
| 19-02-2021 02:13 |
IBdaMann ★★★★★ ★★★★★
(14389) |
Spongy Iris wrote: Because the stratosphere suddenly warms!
Every day the earth's solid and liquid surface heats air via conduction which then increases the stratosphere's temperature via convection.
... every day ... yet you seem puzzled. Is there something else you need explained to you?
.
I don't think i can [define it]. I just kind of get a feel for the phrase. - keepit
A Spaghetti strainer with the faucet running, retains water- tmiddles
Clouds don't trap heat. Clouds block cold. - Spongy Iris
Printing dollars to pay debt doesn't increase the number of dollars. - keepit
If Venus were a black body it would have a much much lower temperature than what we found there.- tmiddles
Ah the "Valid Data" myth of ITN/IBD. - tmiddles
Ceist - I couldn't agree with you more. But when money and religion are involved, and there are people who value them above all else, then the lies begin. - trafn
You are completely misunderstanding their use of the word "accumulation"! - Climate Scientist.
The Stefan-Boltzman equation doesn't come up with the correct temperature if greenhouse gases are not considered - Hank
:*sigh* Not the "raw data" crap. - Leafsdude
IB STILL hasn't explained what Planck's Law means. Just more hand waving that it applies to everything and more asserting that the greenhouse effect 'violates' it.- Ceist |
| 19-02-2021 03:51 |
Spongy Iris ★★★★☆ ★★★★☆
(1643) |
IBdaMann wrote:
Spongy Iris wrote: Because the stratosphere suddenly warms!
Every day the earth's solid and liquid surface heats air via conduction which then increases the stratosphere's temperature via convection.
... every day ... yet you seem puzzled. Is there something else you need explained to you?
.
Your explanation does not appear to be relevant to the temperature profile you see in the atmosphere, nor to the occurence of SSW in the Arctic in January and/or February every 6 of 10 years.
The air gets colder and colder for about 6 miles up, in the troposphere, and then it starts warming in the stratosphere. So it's not just, "earth's solid and liquid surface heats air via conduction" which is applicable to the temperature profile in the atmosphere.
SSW should be pondered upon, because it is a large temperature increase that occurs in a short period of time, during a time when there is no sun shining on it. Where did the energy to heat up the stratosphere come from?
%20(1).png)
Edited on 19-02-2021 03:53 |
| 19-02-2021 04:31 |
James___★★★★★
(5513) |
Spongy Iris wrote:
IBdaMann wrote:
Spongy Iris wrote: Because the stratosphere suddenly warms!
Every day the earth's solid and liquid surface heats air via conduction which then increases the stratosphere's temperature via convection.
... every day ... yet you seem puzzled. Is there something else you need explained to you?
.
Your explanation does not appear to be relevant to the temperature profile you see in the atmosphere, nor to the occurence of SSW in the Arctic in January and/or February every 6 of 10 years.
The air gets colder and colder for about 6 miles up, in the troposphere, and then it starts warming in the stratosphere. So it's not just, "earth's solid and liquid surface heats air via conduction" which is applicable to the temperature profile in the atmosphere.
SSW should be pondered upon, because it is a large temperature increase that occurs in a short period of time, during a time when there is no sun shining on it. Where did the energy to heat up the stratosphere come from?
This might help my hypothesis of CO2 + H2O > CH2O + O2. |
| 19-02-2021 05:54 |
IBdaMann ★★★★★ ★★★★★
(14389) |
Spongy Iris wrote:Your explanation does not appear to be relevant to the temperature profile you see in the atmosphere, nor to the occurence of SSW in the Arctic in January and/or February every 6 of 10 years.
1. I simply rattled off the physics involved. Everything I wrote is necessarily relevant.
2. You have no rational basis for believing in your imaginary "temperature profile" that somehow comes about by virtue of gibberbabble that you also imagine.
Spongy Iris wrote: The air gets colder and colder for about 6 miles up, in the troposphere, and then it starts warming in the stratosphere.
Yes. The height of the troposphere varies depending on proximity to the equator but the troposphere is essentially the range of earth's natural "ground effect" and is why almost all weather occurs therein.
It's the same principle as ice water in a pot on the stove. The solid and ocean surface heat the bottom of the atmopshere and the now warm air rises. The cold air at the top of the troposphere descends and displaces the rising warm air creating a cycle. If there is a sufficient amount of cold air at any given moment, a storm will form, e.g. a tornado, a hurricane, etc.. The cold air that reaches the surface becomes heated and rises perpetuating the turbulence that gives us our weather.
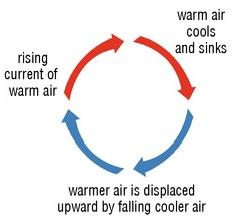
As I said, this cycle is as high as it is and defines the troposphere. Naturally this ceases at the top of the toposphere (by definition). We call the point that the stratosphere begins the "tropopause."
Spongy Iris wrote: So it's not just, "earth's solid and liquid surface heats air via conduction" which is applicable to the temperature profile in the atmosphere.
OK, you got me. I only listed half of the cycle. Above in this post I just spelled out the other half.
Spongy Iris wrote:Where did the energy to heat up the stratosphere come from?
There is far less atmosphere in the stratosphere so rising in elevation brings you closer and closer to lunar conditions, i.e. you move in the direction of corresponding conditions on the moon. If you are talking about daytime then rising in the stratosphere brings you closer to lunar daytime conditions, i.e. the temperature increases. If you are talking about nighttime then rising in the stratosphere brings you closer to lunar nighttime, i.e. the temperature decreases.
The daytime mesophere confuses most people because it is normally poorly explained. People tend to simply say that "temperatures begin to decrease as one rises in the mesosphere." This is totally misleading. First, daytime needs to be specified because nighttime mesosphere just keeps dropping in temperature as you rise in elevation. The reason the mesosphere differs from the troposphere and the stratosphere is that there is essentially no atmosphere in what we are calling a layer of the atmosphere, i.e. it becomes its own contradiction. The amount of air and pressure drops close to zero and this has a drastically greater effect on the air's temperature than any actual increase in the air's temperature (the temperature of the individual molecules). Refer to any YouTube video on how to freeze water at room temperature simply by dropping the air pressure sufficiently. This is why the daytime mesosphere appears to decrease in temperature with elevation; it's an air pressure thing, or lack thereof.
The thermosphere is marked by really not having air except for the occasional molecules that happen to float into it. I'm not sure why we call this area a layer of the atmosphere because it's essentially outer space. We put satellites into the thermosphere because we aren't afraid of there being any atmospheric drag.
I personally don't recognize an exosphere. The only air molecules that you'll find in the space above the thermosphere are those that are taking their shot at escaping earth's gravity and as such are at zero pressure. Zero. There is no way to take the temperature of these particular molecules and they might just be floating off into space.
.
I don't think i can [define it]. I just kind of get a feel for the phrase. - keepit
A Spaghetti strainer with the faucet running, retains water- tmiddles
Clouds don't trap heat. Clouds block cold. - Spongy Iris
Printing dollars to pay debt doesn't increase the number of dollars. - keepit
If Venus were a black body it would have a much much lower temperature than what we found there.- tmiddles
Ah the "Valid Data" myth of ITN/IBD. - tmiddles
Ceist - I couldn't agree with you more. But when money and religion are involved, and there are people who value them above all else, then the lies begin. - trafn
You are completely misunderstanding their use of the word "accumulation"! - Climate Scientist.
The Stefan-Boltzman equation doesn't come up with the correct temperature if greenhouse gases are not considered - Hank
:*sigh* Not the "raw data" crap. - Leafsdude
IB STILL hasn't explained what Planck's Law means. Just more hand waving that it applies to everything and more asserting that the greenhouse effect 'violates' it.- Ceist |
| 19-02-2021 06:23 |
IBdaMann ★★★★★ ★★★★★
(14389) |
James___ wrote: This might help my hypothesis of CO2 + H2O > CH2O + O2.
James__ ,
CO2 + H2O = Carbonated Water.
.
Attached image:
 |
|
| 19-02-2021 08:47 |
James___★★★★★
(5513) |
IBdaMann wrote:
James___ wrote: This might help my hypothesis of CO2 + H2O > CH2O + O2.
James__ ,
CO2 + H2O = Carbonated Water.
.
Now that's classy. |
| 19-02-2021 08:52 |
James___★★★★★
(5513) |
Spongy Iris wrote:
IBdaMann wrote:
Spongy Iris wrote: Because the stratosphere suddenly warms!
Every day the earth's solid and liquid surface heats air via conduction which then increases the stratosphere's temperature via convection.
... every day ... yet you seem puzzled. Is there something else you need explained to you?
.
Your explanation does not appear to be relevant to the temperature profile you see in the atmosphere, nor to the occurence of SSW in the Arctic in January and/or February every 6 of 10 years.
The air gets colder and colder for about 6 miles up, in the troposphere, and then it starts warming in the stratosphere. So it's not just, "earth's solid and liquid surface heats air via conduction" which is applicable to the temperature profile in the atmosphere.
SSW should be pondered upon, because it is a large temperature increase that occurs in a short period of time, during a time when there is no sun shining on it. Where did the energy to heat up the stratosphere come from?
With ozone, if it circulates to where there is no solar radiation then it will conserve its energy longer. They say incoming solar IR is why O3 > O & O2.
Since the Earth has a magnetic field, It's possible it could be absorbing some of that energy as well.
To have a bit of fun with this, December 21st is the winter solstice in the northern hemisphere. About January 23rd the Earth is it's furthest distance from the Sun.
With the winter solstice in the southern hemisphere, the Earth isn't as far away. The question might be, where did all of the ozone come from?
And with Texas, when O & O2 + hv > O3, how much energy is really being kept out of the atmosphere?
Edited on 19-02-2021 09:34 |
| 19-02-2021 12:22 |
duncan61★★★★★
(2021) |
I have tried for 2 nights to find a picture of the North pole how it is right now and all I can get is old stuff.There must be some recent pictures somewhere can someone find something |
| 19-02-2021 15:10 |
James___★★★★★
(5513) |
duncan61 wrote:
I have tried for 2 nights to find a picture of the North pole how it is right now and all I can get is old stuff.There must be some recent pictures somewhere can someone find something
I use the search term current ozone arctic. What I've posted is from one NASA web page. I don't know what other agencies or different countries might put online. At the top on the right side of the page, it will say northern or southern hemisphere. It's to go to the other hemisphere.
With monitoring the ozone layer, it might be who has a satellite or satellites that monitor ozone levels.
https://ozonewatch.gsfc.nasa.gov/NH.html |
| 19-02-2021 22:57 |
Spongy Iris ★★★★☆ ★★★★☆
(1643) |
James___ wrote:
Spongy Iris wrote:
IBdaMann wrote:
Spongy Iris wrote: Because the stratosphere suddenly warms!
Every day the earth's solid and liquid surface heats air via conduction which then increases the stratosphere's temperature via convection.
... every day ... yet you seem puzzled. Is there something else you need explained to you?
.
Your explanation does not appear to be relevant to the temperature profile you see in the atmosphere, nor to the occurence of SSW in the Arctic in January and/or February every 6 of 10 years.
The air gets colder and colder for about 6 miles up, in the troposphere, and then it starts warming in the stratosphere. So it's not just, "earth's solid and liquid surface heats air via conduction" which is applicable to the temperature profile in the atmosphere.
SSW should be pondered upon, because it is a large temperature increase that occurs in a short period of time, during a time when there is no sun shining on it. Where did the energy to heat up the stratosphere come from?
This might help my hypothesis of CO2 + H2O > CH2O + O2.
Yes I heard it from you first there is formaldehyde in the atmosphere.
There requires a lot of energy to produce such a reaction you described, yes?
I think the formaldehyde in the atmosphere is naturally occurring...
%20(1).png) |
| 19-02-2021 23:00 |
Spongy Iris ★★★★☆ ★★★★☆
(1643) |
James___ wrote:
Spongy Iris wrote:
IBdaMann wrote:
Spongy Iris wrote: Because the stratosphere suddenly warms!
Every day the earth's solid and liquid surface heats air via conduction which then increases the stratosphere's temperature via convection.
... every day ... yet you seem puzzled. Is there something else you need explained to you?
.
Your explanation does not appear to be relevant to the temperature profile you see in the atmosphere, nor to the occurence of SSW in the Arctic in January and/or February every 6 of 10 years.
The air gets colder and colder for about 6 miles up, in the troposphere, and then it starts warming in the stratosphere. So it's not just, "earth's solid and liquid surface heats air via conduction" which is applicable to the temperature profile in the atmosphere.
SSW should be pondered upon, because it is a large temperature increase that occurs in a short period of time, during a time when there is no sun shining on it. Where did the energy to heat up the stratosphere come from?
With ozone, if it circulates to where there is no solar radiation then it will conserve its energy longer. They say incoming solar IR is why O3 > O & O2.
Since the Earth has a magnetic field, It's possible it could be absorbing some of that energy as well.
To have a bit of fun with this, December 21st is the winter solstice in the northern hemisphere. About January 23rd the Earth is it's furthest distance from the Sun.
With the winter solstice in the southern hemisphere, the Earth isn't as far away. The question might be, where did all of the ozone come from?
And with Texas, when O & O2 + hv > O3, how much energy is really being kept out of the atmosphere?
Idk man. I'm having a heard time believing the ozone which blew up from bottom to the top of the world can warm the stratosphere by 50 K in 1 week.
%20(1).png) |
| 19-02-2021 23:45 |
IBdaMann ★★★★★ ★★★★★
(14389) |
Spongy Iris wrote:Idk man. I'm having a heard time believing the ozone which blew up from bottom to the top of the world can warm the stratosphere by 50 K in 1 week.
It is good that you are having a tough time believing that. There's no reason to believe such nonsense. I wouldn't believe it if someone were to try to pawn that off on me.
Well done.
.
I don't think i can [define it]. I just kind of get a feel for the phrase. - keepit
A Spaghetti strainer with the faucet running, retains water- tmiddles
Clouds don't trap heat. Clouds block cold. - Spongy Iris
Printing dollars to pay debt doesn't increase the number of dollars. - keepit
If Venus were a black body it would have a much much lower temperature than what we found there.- tmiddles
Ah the "Valid Data" myth of ITN/IBD. - tmiddles
Ceist - I couldn't agree with you more. But when money and religion are involved, and there are people who value them above all else, then the lies begin. - trafn
You are completely misunderstanding their use of the word "accumulation"! - Climate Scientist.
The Stefan-Boltzman equation doesn't come up with the correct temperature if greenhouse gases are not considered - Hank
:*sigh* Not the "raw data" crap. - Leafsdude
IB STILL hasn't explained what Planck's Law means. Just more hand waving that it applies to everything and more asserting that the greenhouse effect 'violates' it.- Ceist |
| 19-02-2021 23:48 |
James___★★★★★
(5513) |
Spongy Iris wrote:
James___ wrote:
Spongy Iris wrote:
IBdaMann wrote:
Spongy Iris wrote: Because the stratosphere suddenly warms!
Every day the earth's solid and liquid surface heats air via conduction which then increases the stratosphere's temperature via convection.
... every day ... yet you seem puzzled. Is there something else you need explained to you?
.
Your explanation does not appear to be relevant to the temperature profile you see in the atmosphere, nor to the occurence of SSW in the Arctic in January and/or February every 6 of 10 years.
The air gets colder and colder for about 6 miles up, in the troposphere, and then it starts warming in the stratosphere. So it's not just, "earth's solid and liquid surface heats air via conduction" which is applicable to the temperature profile in the atmosphere.
SSW should be pondered upon, because it is a large temperature increase that occurs in a short period of time, during a time when there is no sun shining on it. Where did the energy to heat up the stratosphere come from?
With ozone, if it circulates to where there is no solar radiation then it will conserve its energy longer. They say incoming solar IR is why O3 > O & O2.
Since the Earth has a magnetic field, It's possible it could be absorbing some of that energy as well.
To have a bit of fun with this, December 21st is the winter solstice in the northern hemisphere. About January 23rd the Earth is it's furthest distance from the Sun.
With the winter solstice in the southern hemisphere, the Earth isn't as far away. The question might be, where did all of the ozone come from?
And with Texas, when O & O2 + hv > O3, how much energy is really being kept out of the atmosphere?
Idk man. I'm having a heard time believing the ozone which blew up from bottom to the top of the world can warm the stratosphere by 50 K in 1 week.
I think it came from California starting in January. I'm pursuing having research done to explain this quote of the IPCC in its 2013 climate report. With the abundance of CO2 in the upper latitudes of the northern hemisphere, it would cause a depleted ozone layer in other parts of the atmosphere. No such reports have been made known.
Carbon dioxide (CO2), nitrous oxide (N2O), and methane (CH4) are each important to climate forcing and to the levels of stratospheric ozone (see Chapter 2). In terms of the globally averaged ozone column, additional N2O leads to lower ozone levels, whereas additional CO2 and CH4 lead to higher ozone levels. Ozone depletion to date would have been greater if not for the historical increases in CO2 and CH4.
https://csl.noaa.gov/assessments/ozone/2014/summary/ch5.html
Edited on 19-02-2021 23:49 |
| 19-02-2021 23:55 |
Spongy Iris ★★★★☆ ★★★★☆
(1643) |
IBdaMann wrote:
Spongy Iris wrote:Your explanation does not appear to be relevant to the temperature profile you see in the atmosphere, nor to the occurence of SSW in the Arctic in January and/or February every 6 of 10 years.
1. I simply rattled off the physics involved. Everything I wrote is necessarily relevant.
2. You have no rational basis for believing in your imaginary "temperature profile" that somehow comes about by virtue of gibberbabble that you also imagine.
Spongy Iris wrote: The air gets colder and colder for about 6 miles up, in the troposphere, and then it starts warming in the stratosphere.
Yes. The height of the troposphere varies depending on proximity to the equator but the troposphere is essentially the range of earth's natural "ground effect" and is why almost all weather occurs therein.
It's the same principle as ice water in a pot on the stove. The solid and ocean surface heat the bottom of the atmopshere and the now warm air rises. The cold air at the top of the troposphere descends and displaces the rising warm air creating a cycle. If there is a sufficient amount of cold air at any given moment, a storm will form, e.g. a tornado, a hurricane, etc.. The cold air that reaches the surface becomes heated and rises perpetuating the turbulence that gives us our weather.

As I said, this cycle is as high as it is and defines the troposphere. Naturally this ceases at the top of the toposphere (by definition). We call the point that the stratosphere begins the "tropopause."
Spongy Iris wrote: So it's not just, "earth's solid and liquid surface heats air via conduction" which is applicable to the temperature profile in the atmosphere.
OK, you got me. I only listed half of the cycle. Above in this post I just spelled out the other half.
Spongy Iris wrote:Where did the energy to heat up the stratosphere come from?
There is far less atmosphere in the stratosphere so rising in elevation brings you closer and closer to lunar conditions, i.e. you move in the direction of corresponding conditions on the moon. If you are talking about daytime then rising in the stratosphere brings you closer to lunar daytime conditions, i.e. the temperature increases. If you are talking about nighttime then rising in the stratosphere brings you closer to lunar nighttime, i.e. the temperature decreases.
The daytime mesophere confuses most people because it is normally poorly explained. People tend to simply say that "temperatures begin to decrease as one rises in the mesosphere." This is totally misleading. First, daytime needs to be specified because nighttime mesosphere just keeps dropping in temperature as you rise in elevation. The reason the mesosphere differs from the troposphere and the stratosphere is that there is essentially no atmosphere in what we are calling a layer of the atmosphere, i.e. it becomes its own contradiction. The amount of air and pressure drops close to zero and this has a drastically greater effect on the air's temperature than any actual increase in the air's temperature (the temperature of the individual molecules). Refer to any YouTube video on how to freeze water at room temperature simply by dropping the air pressure sufficiently. This is why the daytime mesosphere appears to decrease in temperature with elevation; it's an air pressure thing, or lack thereof.
The thermosphere is marked by really not having air except for the occasional molecules that happen to float into it. I'm not sure why we call this area a layer of the atmosphere because it's essentially outer space. We put satellites into the thermosphere because we aren't afraid of there being any atmospheric drag.
I personally don't recognize an exosphere. The only air molecules that you'll find in the space above the thermosphere are those that are taking their shot at escaping earth's gravity and as such are at zero pressure. Zero. There is no way to take the temperature of these particular molecules and they might just be floating off into space.
.
You cannot explain the wild swings in the temperature profile of the atmosphere with the information you are able to provide. Go ahead and just deny the following fact because that is the only point you can make. Everything else you say is beside the point.

These wild temperature swings must be known because they can be assessed using satellite technology.
%20(1).png) |
| 20-02-2021 00:19 |
James___★★★★★
(5513) |
Spongy Iris wrote:
James___ wrote:
Spongy Iris wrote:
IBdaMann wrote:
Spongy Iris wrote: Because the stratosphere suddenly warms!
Every day the earth's solid and liquid surface heats air via conduction which then increases the stratosphere's temperature via convection.
... every day ... yet you seem puzzled. Is there something else you need explained to you?
.
Your explanation does not appear to be relevant to the temperature profile you see in the atmosphere, nor to the occurence of SSW in the Arctic in January and/or February every 6 of 10 years.
The air gets colder and colder for about 6 miles up, in the troposphere, and then it starts warming in the stratosphere. So it's not just, "earth's solid and liquid surface heats air via conduction" which is applicable to the temperature profile in the atmosphere.
SSW should be pondered upon, because it is a large temperature increase that occurs in a short period of time, during a time when there is no sun shining on it. Where did the energy to heat up the stratosphere come from?
This might help my hypothesis of CO2 + H2O > CH2O + O2.
Yes I heard it from you first there is formaldehyde in the atmosphere.
There requires a lot of energy to produce such a reaction you described, yes?
I think the formaldehyde in the atmosphere is naturally occurring...
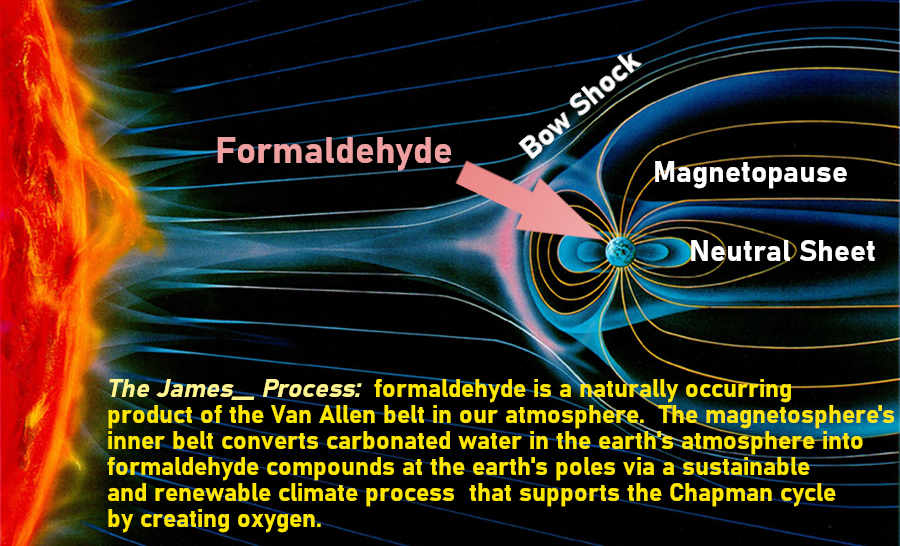
I think it is. What I think scientists overlook is the role that the Van Allen radiation belts play in our atmosphere. They in a sense are a static charge.
An example is this video showing a static charge changes the flow of water.
https://www.youtube.com/watch?v=VhWQ-r1LYXY&t=1s
The reason this happens is that water is "polar". Thus it is attracted to the static electricity. Gasses in our atmosphere like water vapor, CO2, the oxygen element and ozone are also polar. And with formaldehyde, it is a polar compound.
This suggests that the Van Allen radiation belts can attract such molecules/compounds. At the same time this would also cause them to become more excited. This IMO is what would allow for CO2 and H2O (both polar molecules) to interact and form formaldehyde. This in turn would release a non-polar O2 molecule which could support the Chapman cycle (the ozone layer).
The German historical project that I am working on, if successful would help to make known what I think about the IPCC report and what experiment might help to demonstrate a connection between observation and what physics allows for.
By early next week I should be able to post a picture of it (in the thread German Historical Project) mostly complete. I will be doing a test to see if torque can be conserved as momentum. If successful then I will be hopeful.
Edited on 20-02-2021 00:25 |
| 20-02-2021 04:54 |
IBdaMann ★★★★★ ★★★★★
(14389) |
James___ wrote:Spongy Iris wrote:I think the formaldehyde in the atmosphere is naturally occurring... I think it is. What I think scientists overlook is the role that the Van Allen radiation belts play in our atmosphere. They in a sense are a static charge.
An example is this video showing a static charge changes the flow of water.
https://www.youtube.com/watch?v=VhWQ-r1LYXY&t=1s
The reason this happens is that water is "polar". Thus it is attracted to the static electricity. Gasses in our atmosphere like water vapor, CO2, the oxygen element and ozone are also polar. And with formaldehyde, it is a polar compound.
This suggests that the Van Allen radiation belts can attract such molecules/compounds. At the same time this would also cause them to become more excited. This IMO is what would allow for CO2 and H2O (both polar molecules) to interact and form formaldehyde. This in turn would release a non-polar O2 molecule which could support the Chapman cycle (the ozone layer).
The German historical project that I am working on, if successful would help to make known what I think about the IPCC report and what experiment might help to demonstrate a connection between observation and what physics allows for.
By early next week I should be able to post a picture of it (in the thread German Historical Project) mostly complete. I will be doing a test to see if torque can be conserved as momentum. If successful then I will be hopeful.
.
Attached image:
 |
| 20-02-2021 05:17 |
James___★★★★★
(5513) |
IBdaMann wrote:
James___ wrote:Spongy Iris wrote:I think the formaldehyde in the atmosphere is naturally occurring... I think it is. What I think scientists overlook is the role that the Van Allen radiation belts play in our atmosphere. They in a sense are a static charge.
An example is this video showing a static charge changes the flow of water.
https://www.youtube.com/watch?v=VhWQ-r1LYXY&t=1s
The reason this happens is that water is "polar". Thus it is attracted to the static electricity. Gasses in our atmosphere like water vapor, CO2, the oxygen element and ozone are also polar. And with formaldehyde, it is a polar compound.
This suggests that the Van Allen radiation belts can attract such molecules/compounds. At the same time this would also cause them to become more excited. This IMO is what would allow for CO2 and H2O (both polar molecules) to interact and form formaldehyde. This in turn would release a non-polar O2 molecule which could support the Chapman cycle (the ozone layer).
The German historical project that I am working on, if successful would help to make known what I think about the IPCC report and what experiment might help to demonstrate a connection between observation and what physics allows for.
By early next week I should be able to post a picture of it (in the thread German Historical Project) mostly complete. I will be doing a test to see if torque can be conserved as momentum. If successful then I will be hopeful.
.
IBDM, Thank You for posting that. I think everyone will notice that the Earth's magnetic field is CLOSER to the Earth when it's facing the solar wind. And as the solar wind sails past the Earth, it stretches the Earth's magnetic field over a greater distance.
You helped to illustrate a point that I wanted to make. When the Earth's magnetic field is closer to it, it can excite molecules in the atmosphere more easily. And CH2O or as you would say to be scientifically correct, HCHO only occurs during the day and never at night.
It's very bright of you Sun to observe that. And as you so correctly stated Sun, solar radiation is a photolytic process which breaks down molecules. And in this instance, the inverse happens. Why I call you Sun son, you are that bright.
Attached image:
Edited on 20-02-2021 05:19 |
| 20-02-2021 08:31 |
HarveyH55 ★★★★★ ★★★★★
(5196) |
Wow, I only knew about formaldehyde from biologic sources, released as part of the decomposition process. |
| 20-02-2021 16:19 |
Into the Night ★★★★★ ★★★★★
(21582) |
James___ wrote:
Spongy Iris wrote:
IBdaMann wrote:
Spongy Iris wrote: Because the stratosphere suddenly warms!
Every day the earth's solid and liquid surface heats air via conduction which then increases the stratosphere's temperature via convection.
... every day ... yet you seem puzzled. Is there something else you need explained to you?
.
Your explanation does not appear to be relevant to the temperature profile you see in the atmosphere, nor to the occurence of SSW in the Arctic in January and/or February every 6 of 10 years.
The air gets colder and colder for about 6 miles up, in the troposphere, and then it starts warming in the stratosphere. So it's not just, "earth's solid and liquid surface heats air via conduction" which is applicable to the temperature profile in the atmosphere.
SSW should be pondered upon, because it is a large temperature increase that occurs in a short period of time, during a time when there is no sun shining on it. Where did the energy to heat up the stratosphere come from?
With ozone, if it circulates to where there is no solar radiation then it will conserve its energy longer. They say incoming solar IR is why O3 > O & O2.
Infrared light does not create or destroy ozone.
James___ wrote:
Since the Earth has a magnetic field, It's possible it could be absorbing some of that energy as well.
A magnetic field is not energy.
James___ wrote:
To have a bit of fun with this, December 21st is the winter solstice in the northern hemisphere. About January 23rd the Earth is it's furthest distance from the Sun.
Jan 3rd this year was peihelion (Earth's closest approach to the Sun). July 4th is aphelion (Earth's furthest distance from the Sun). These dates are for 2021.
James___ wrote:
With the winter solstice in the southern hemisphere, the Earth isn't as far away. The question might be, where did all of the ozone come from?
See the Chapman cycle.
James___ wrote:
And with Texas, when O & O2 + hv > O3, how much energy is really being kept out of the atmosphere?
Zero.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 20-02-2021 16:28 |
Into the Night ★★★★★ ★★★★★
(21582) |
Spongy Iris wrote:
IBdaMann wrote:
Spongy Iris wrote:Your explanation does not appear to be relevant to the temperature profile you see in the atmosphere, nor to the occurence of SSW in the Arctic in January and/or February every 6 of 10 years.
1. I simply rattled off the physics involved. Everything I wrote is necessarily relevant.
2. You have no rational basis for believing in your imaginary "temperature profile" that somehow comes about by virtue of gibberbabble that you also imagine.
Spongy Iris wrote: The air gets colder and colder for about 6 miles up, in the troposphere, and then it starts warming in the stratosphere.
Yes. The height of the troposphere varies depending on proximity to the equator but the troposphere is essentially the range of earth's natural "ground effect" and is why almost all weather occurs therein.
It's the same principle as ice water in a pot on the stove. The solid and ocean surface heat the bottom of the atmopshere and the now warm air rises. The cold air at the top of the troposphere descends and displaces the rising warm air creating a cycle. If there is a sufficient amount of cold air at any given moment, a storm will form, e.g. a tornado, a hurricane, etc.. The cold air that reaches the surface becomes heated and rises perpetuating the turbulence that gives us our weather.

As I said, this cycle is as high as it is and defines the troposphere. Naturally this ceases at the top of the toposphere (by definition). We call the point that the stratosphere begins the "tropopause."
Spongy Iris wrote: So it's not just, "earth's solid and liquid surface heats air via conduction" which is applicable to the temperature profile in the atmosphere.
OK, you got me. I only listed half of the cycle. Above in this post I just spelled out the other half.
Spongy Iris wrote:Where did the energy to heat up the stratosphere come from?
There is far less atmosphere in the stratosphere so rising in elevation brings you closer and closer to lunar conditions, i.e. you move in the direction of corresponding conditions on the moon. If you are talking about daytime then rising in the stratosphere brings you closer to lunar daytime conditions, i.e. the temperature increases. If you are talking about nighttime then rising in the stratosphere brings you closer to lunar nighttime, i.e. the temperature decreases.
The daytime mesophere confuses most people because it is normally poorly explained. People tend to simply say that "temperatures begin to decrease as one rises in the mesosphere." This is totally misleading. First, daytime needs to be specified because nighttime mesosphere just keeps dropping in temperature as you rise in elevation. The reason the mesosphere differs from the troposphere and the stratosphere is that there is essentially no atmosphere in what we are calling a layer of the atmosphere, i.e. it becomes its own contradiction. The amount of air and pressure drops close to zero and this has a drastically greater effect on the air's temperature than any actual increase in the air's temperature (the temperature of the individual molecules). Refer to any YouTube video on how to freeze water at room temperature simply by dropping the air pressure sufficiently. This is why the daytime mesosphere appears to decrease in temperature with elevation; it's an air pressure thing, or lack thereof.
The thermosphere is marked by really not having air except for the occasional molecules that happen to float into it. I'm not sure why we call this area a layer of the atmosphere because it's essentially outer space. We put satellites into the thermosphere because we aren't afraid of there being any atmospheric drag.
I personally don't recognize an exosphere. The only air molecules that you'll find in the space above the thermosphere are those that are taking their shot at escaping earth's gravity and as such are at zero pressure. Zero. There is no way to take the temperature of these particular molecules and they might just be floating off into space.
.
You cannot explain the wild swings in the temperature profile of the atmosphere with the information you are able to provide. Go ahead and just deny the following fact because that is the only point you can make. Everything else you say is beside the point.

These wild temperature swings must be known because they can be assessed using satellite technology.
This temperature profile is not determined by satellites, but by weather balloons and rocket launches. It is a generic profile. The actual temperature at any altitude in the atmosphere is unknown.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
|
| 20-02-2021 20:03 |
Spongy Iris ★★★★☆ ★★★★☆
(1643) |
Into the Night wrote:
Spongy Iris wrote:
IBdaMann wrote:
Spongy Iris wrote:Your explanation does not appear to be relevant to the temperature profile you see in the atmosphere, nor to the occurence of SSW in the Arctic in January and/or February every 6 of 10 years.
1. I simply rattled off the physics involved. Everything I wrote is necessarily relevant.
2. You have no rational basis for believing in your imaginary "temperature profile" that somehow comes about by virtue of gibberbabble that you also imagine.
Spongy Iris wrote: The air gets colder and colder for about 6 miles up, in the troposphere, and then it starts warming in the stratosphere.
Yes. The height of the troposphere varies depending on proximity to the equator but the troposphere is essentially the range of earth's natural "ground effect" and is why almost all weather occurs therein.
It's the same principle as ice water in a pot on the stove. The solid and ocean surface heat the bottom of the atmopshere and the now warm air rises. The cold air at the top of the troposphere descends and displaces the rising warm air creating a cycle. If there is a sufficient amount of cold air at any given moment, a storm will form, e.g. a tornado, a hurricane, etc.. The cold air that reaches the surface becomes heated and rises perpetuating the turbulence that gives us our weather.

As I said, this cycle is as high as it is and defines the troposphere. Naturally this ceases at the top of the toposphere (by definition). We call the point that the stratosphere begins the "tropopause."
Spongy Iris wrote: So it's not just, "earth's solid and liquid surface heats air via conduction" which is applicable to the temperature profile in the atmosphere.
OK, you got me. I only listed half of the cycle. Above in this post I just spelled out the other half.
Spongy Iris wrote:Where did the energy to heat up the stratosphere come from?
There is far less atmosphere in the stratosphere so rising in elevation brings you closer and closer to lunar conditions, i.e. you move in the direction of corresponding conditions on the moon. If you are talking about daytime then rising in the stratosphere brings you closer to lunar daytime conditions, i.e. the temperature increases. If you are talking about nighttime then rising in the stratosphere brings you closer to lunar nighttime, i.e. the temperature decreases.
The daytime mesophere confuses most people because it is normally poorly explained. People tend to simply say that "temperatures begin to decrease as one rises in the mesosphere." This is totally misleading. First, daytime needs to be specified because nighttime mesosphere just keeps dropping in temperature as you rise in elevation. The reason the mesosphere differs from the troposphere and the stratosphere is that there is essentially no atmosphere in what we are calling a layer of the atmosphere, i.e. it becomes its own contradiction. The amount of air and pressure drops close to zero and this has a drastically greater effect on the air's temperature than any actual increase in the air's temperature (the temperature of the individual molecules). Refer to any YouTube video on how to freeze water at room temperature simply by dropping the air pressure sufficiently. This is why the daytime mesosphere appears to decrease in temperature with elevation; it's an air pressure thing, or lack thereof.
The thermosphere is marked by really not having air except for the occasional molecules that happen to float into it. I'm not sure why we call this area a layer of the atmosphere because it's essentially outer space. We put satellites into the thermosphere because we aren't afraid of there being any atmospheric drag.
I personally don't recognize an exosphere. The only air molecules that you'll find in the space above the thermosphere are those that are taking their shot at escaping earth's gravity and as such are at zero pressure. Zero. There is no way to take the temperature of these particular molecules and they might just be floating off into space.
.
You cannot explain the wild swings in the temperature profile of the atmosphere with the information you are able to provide. Go ahead and just deny the following fact because that is the only point you can make. Everything else you say is beside the point.

These wild temperature swings must be known because they can be assessed using satellite technology.
This temperature profile is not determined by satellites, but by weather balloons and rocket launches. It is a generic profile. The actual temperature at any altitude in the atmosphere is unknown.
The general ranges in temperature is a known fact.
%20(1).png) |
| 20-02-2021 20:17 |
Spongy Iris ★★★★☆ ★★★★☆
(1643) |
James___ wrote:
IBdaMann wrote:
James___ wrote:Spongy Iris wrote:I think the formaldehyde in the atmosphere is naturally occurring... I think it is. What I think scientists overlook is the role that the Van Allen radiation belts play in our atmosphere. They in a sense are a static charge.
An example is this video showing a static charge changes the flow of water.
https://www.youtube.com/watch?v=VhWQ-r1LYXY&t=1s
The reason this happens is that water is "polar". Thus it is attracted to the static electricity. Gasses in our atmosphere like water vapor, CO2, the oxygen element and ozone are also polar. And with formaldehyde, it is a polar compound.
This suggests that the Van Allen radiation belts can attract such molecules/compounds. At the same time this would also cause them to become more excited. This IMO is what would allow for CO2 and H2O (both polar molecules) to interact and form formaldehyde. This in turn would release a non-polar O2 molecule which could support the Chapman cycle (the ozone layer).
The German historical project that I am working on, if successful would help to make known what I think about the IPCC report and what experiment might help to demonstrate a connection between observation and what physics allows for.
By early next week I should be able to post a picture of it (in the thread German Historical Project) mostly complete. I will be doing a test to see if torque can be conserved as momentum. If successful then I will be hopeful.
.
IBDM, Thank You for posting that. I think everyone will notice that the Earth's magnetic field is CLOSER to the Earth when it's facing the solar wind. And as the solar wind sails past the Earth, it stretches the Earth's magnetic field over a greater distance.
You helped to illustrate a point that I wanted to make. When the Earth's magnetic field is closer to it, it can excite molecules in the atmosphere more easily. And CH2O or as you would say to be scientifically correct, HCHO only occurs during the day and never at night.
It's very bright of you Sun to observe that. And as you so correctly stated Sun, solar radiation is a photolytic process which breaks down molecules. And in this instance, the inverse happens. Why I call you Sun son, you are that bright.
I was actually thinking kind of the same thing as your Sun.
It seems like ozone and formaldehyde are originating from around 30 miles altitude. But the Van Allen belts I read don't start until around 400 miles altitude.
%20(1).png) |
| 20-02-2021 20:43 |
Into the Night ★★★★★ ★★★★★
(21582) |
Spongy Iris wrote:
James___ wrote:
IBdaMann wrote:
James___ wrote:Spongy Iris wrote:I think the formaldehyde in the atmosphere is naturally occurring... I think it is. What I think scientists overlook is the role that the Van Allen radiation belts play in our atmosphere. They in a sense are a static charge.
An example is this video showing a static charge changes the flow of water.
https://www.youtube.com/watch?v=VhWQ-r1LYXY&t=1s
The reason this happens is that water is "polar". Thus it is attracted to the static electricity. Gasses in our atmosphere like water vapor, CO2, the oxygen element and ozone are also polar. And with formaldehyde, it is a polar compound.
This suggests that the Van Allen radiation belts can attract such molecules/compounds. At the same time this would also cause them to become more excited. This IMO is what would allow for CO2 and H2O (both polar molecules) to interact and form formaldehyde. This in turn would release a non-polar O2 molecule which could support the Chapman cycle (the ozone layer).
The German historical project that I am working on, if successful would help to make known what I think about the IPCC report and what experiment might help to demonstrate a connection between observation and what physics allows for.
By early next week I should be able to post a picture of it (in the thread German Historical Project) mostly complete. I will be doing a test to see if torque can be conserved as momentum. If successful then I will be hopeful.
.
IBDM, Thank You for posting that. I think everyone will notice that the Earth's magnetic field is CLOSER to the Earth when it's facing the solar wind. And as the solar wind sails past the Earth, it stretches the Earth's magnetic field over a greater distance.
You helped to illustrate a point that I wanted to make. When the Earth's magnetic field is closer to it, it can excite molecules in the atmosphere more easily. And CH2O or as you would say to be scientifically correct, HCHO only occurs during the day and never at night.
It's very bright of you Sun to observe that. And as you so correctly stated Sun, solar radiation is a photolytic process which breaks down molecules. And in this instance, the inverse happens. Why I call you Sun son, you are that bright.
I was actually thinking kind of the same thing as your Sun.
It seems like ozone and formaldehyde are originating from around 30 miles altitude. But the Van Allen belts I read don't start until around 400 miles altitude.
Most ozone forms at around 35000 and up. This is the ozone layer. See the Chapman cycle.
Ozone also forms in the troposphere due to storm activity, and even at the surface.
The Parrot Killer
Debunked in my sig. - tmiddles
Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit
nuclear powered ships do not require nuclear fuel. - Swan
While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan
Edited on 20-02-2021 20:44 |
| 20-02-2021 21:13 |
James___★★★★★
(5513) |
Spongy Iris wrote:
James___ wrote:
IBdaMann wrote:
James___ wrote:Spongy Iris wrote:I think the formaldehyde in the atmosphere is naturally occurring... I think it is. What I think scientists overlook is the role that the Van Allen radiation belts play in our atmosphere. They in a sense are a static charge.
An example is this video showing a static charge changes the flow of water.
https://www.youtube.com/watch?v=VhWQ-r1LYXY&t=1s
The reason this happens is that water is "polar". Thus it is attracted to the static electricity. Gasses in our atmosphere like water vapor, CO2, the oxygen element and ozone are also polar. And with formaldehyde, it is a polar compound.
This suggests that the Van Allen radiation belts can attract such molecules/compounds. At the same time this would also cause them to become more excited. This IMO is what would allow for CO2 and H2O (both polar molecules) to interact and form formaldehyde. This in turn would release a non-polar O2 molecule which could support the Chapman cycle (the ozone layer).
The German historical project that I am working on, if successful would help to make known what I think about the IPCC report and what experiment might help to demonstrate a connection between observation and what physics allows for.
By early next week I should be able to post a picture of it (in the thread German Historical Project) mostly complete. I will be doing a test to see if torque can be conserved as momentum. If successful then I will be hopeful.
.
IBDM, Thank You for posting that. I think everyone will notice that the Earth's magnetic field is CLOSER to the Earth when it's facing the solar wind. And as the solar wind sails past the Earth, it stretches the Earth's magnetic field over a greater distance.
You helped to illustrate a point that I wanted to make. When the Earth's magnetic field is closer to it, it can excite molecules in the atmosphere more easily. And CH2O or as you would say to be scientifically correct, HCHO only occurs during the day and never at night.
It's very bright of you Sun to observe that. And as you so correctly stated Sun, solar radiation is a photolytic process which breaks down molecules. And in this instance, the inverse happens. Why I call you Sun son, you are that bright.
I was actually thinking kind of the same thing as your Sun.
It seems like ozone and formaldehyde are originating from around 30 miles altitude. But the Van Allen belts I read don't start until around 400 miles altitude.
There are no gasses in the stratosphere that would allow either formaldehyde or ozone to occur naturally. Why I say at the top of the troposphere.
I've also read a lot of research (online) that has been done since the 1970's on formaldehyde in the atmosphere.
Edited on 20-02-2021 21:25 |
| 20-02-2021 22:39 |
IBdaMann ★★★★★ ★★★★★
(14389) |
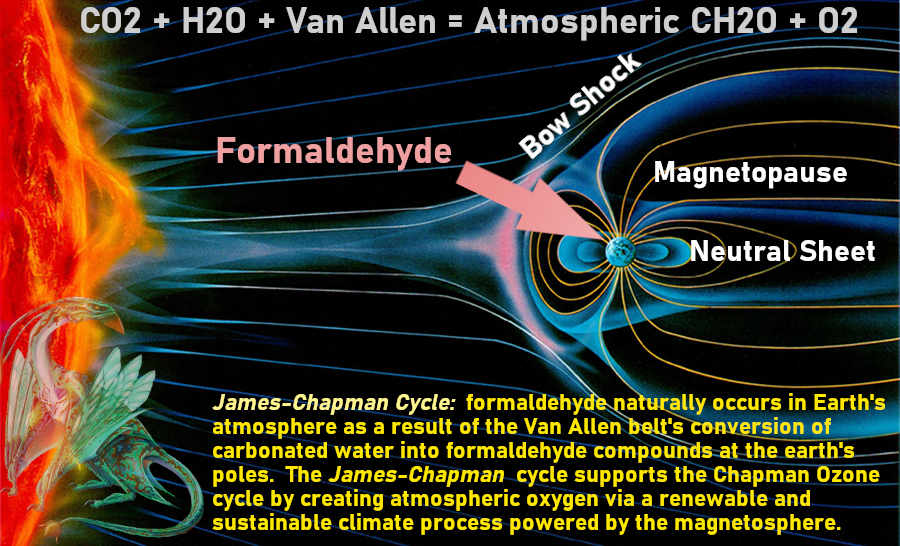
James___ wrote:There are no gasses in the stratosphere that would allow either formaldehyde or ozone to occur naturally. Why I say at the top of the troposphere.
I've also read a lot of research (online) that has been done since the 1970's on formaldehyde in the atmosphere.
Well done James. I have updated my previous hastily-crafted graphic. Here's the new one:
.
Attached image:
 |
| 21-02-2021 00:12 |
Spongy Iris ★★★★☆ ★★★★☆
(1643) |
Into the Night wrote:
Spongy Iris wrote:
James___ wrote:
IBdaMann wrote:
James___ wrote:Spongy Iris wrote:I think the formaldehyde in the atmosphere is naturally occurring... I think it is. What I think scientists overlook is the role that the Van Allen radiation belts play in our atmosphere. They in a sense are a static charge.
An example is this video showing a static charge changes the flow of water.
https://www.youtube.com/watch?v=VhWQ-r1LYXY&t=1s
The reason this happens is that water is "polar". Thus it is attracted to the static electricity. Gasses in our atmosphere like water vapor, CO2, the oxygen element and ozone are also polar. And with formaldehyde, it is a polar compound.
This suggests that the Van Allen radiation belts can attract such molecules/compounds. At the same time this would also cause them to become more excited. This IMO is what would allow for CO2 and H2O (both polar molecules) to interact and form formaldehyde. This in turn would release a non-polar O2 molecule which could support the Chapman cycle (the ozone layer).
The German historical project that I am working on, if successful would help to make known what I think about the IPCC report and what experiment might help to demonstrate a connection between observation and what physics allows for.
By early next week I should be able to post a picture of it (in the thread German Historical Project) mostly complete. I will be doing a test to see if torque can be conserved as momentum. If successful then I will be hopeful.
.
IBDM, Thank You for posting that. I think everyone will notice that the Earth's magnetic field is CLOSER to the Earth when it's facing the solar wind. And as the solar wind sails past the Earth, it stretches the Earth's magnetic field over a greater distance.
You helped to illustrate a point that I wanted to make. When the Earth's magnetic field is closer to it, it can excite molecules in the atmosphere more easily. And CH2O or as you would say to be scientifically correct, HCHO only occurs during the day and never at night.
It's very bright of you Sun to observe that. And as you so correctly stated Sun, solar radiation is a photolytic process which breaks down molecules. And in this instance, the inverse happens. Why I call you Sun son, you are that bright.
I was actually thinking kind of the same thing as your Sun.
It seems like ozone and formaldehyde are originating from around 30 miles altitude. But the Van Allen belts I read don't start until around 400 miles altitude.
Most ozone forms at around 35000 and up. This is the ozone layer. See the Chapman cycle.
Ozone also forms in the troposphere due to storm activity, and even at the surface.
I thought ozone was present from approx 6 to 30 miles altitude in the atmosphere, no?
%20(1).png) |
| 21-02-2021 00:16 |
Spongy Iris ★★★★☆ ★★★★☆
(1643) |
James___ wrote:
Spongy Iris wrote:
James___ wrote:
IBdaMann wrote:
James___ wrote:Spongy Iris wrote:I think the formaldehyde in the atmosphere is naturally occurring... I think it is. What I think scientists overlook is the role that the Van Allen radiation belts play in our atmosphere. They in a sense are a static charge.
An example is this video showing a static charge changes the flow of water.
https://www.youtube.com/watch?v=VhWQ-r1LYXY&t=1s
The reason this happens is that water is "polar". Thus it is attracted to the static electricity. Gasses in our atmosphere like water vapor, CO2, the oxygen element and ozone are also polar. And with formaldehyde, it is a polar compound.
This suggests that the Van Allen radiation belts can attract such molecules/compounds. At the same time this would also cause them to become more excited. This IMO is what would allow for CO2 and H2O (both polar molecules) to interact and form formaldehyde. This in turn would release a non-polar O2 molecule which could support the Chapman cycle (the ozone layer).
The German historical project that I am working on, if successful would help to make known what I think about the IPCC report and what experiment might help to demonstrate a connection between observation and what physics allows for.
By early next week I should be able to post a picture of it (in the thread German Historical Project) mostly complete. I will be doing a test to see if torque can be conserved as momentum. If successful then I will be hopeful.
.
IBDM, Thank You for posting that. I think everyone will notice that the Earth's magnetic field is CLOSER to the Earth when it's facing the solar wind. And as the solar wind sails past the Earth, it stretches the Earth's magnetic field over a greater distance.
You helped to illustrate a point that I wanted to make. When the Earth's magnetic field is closer to it, it can excite molecules in the atmosphere more easily. And CH2O or as you would say to be scientifically correct, HCHO only occurs during the day and never at night.
It's very bright of you Sun to observe that. And as you so correctly stated Sun, solar radiation is a photolytic process which breaks down molecules. And in this instance, the inverse happens. Why I call you Sun son, you are that bright.
I was actually thinking kind of the same thing as your Sun.
It seems like ozone and formaldehyde are originating from around 30 miles altitude. But the Van Allen belts I read don't start until around 400 miles altitude.
There are no gasses in the stratosphere that would allow either formaldehyde or ozone to occur naturally. Why I say at the top of the troposphere.
I've also read a lot of research (online) that has been done since the 1970's on formaldehyde in the atmosphere.
Oh, right, theres no water vapor that high I don't think.
Well now I'm back onto the theory that it is viruses and or bacteria decomposing organic matter in the atmosphere which is causing the formaldehyde. Kind of what Harvey just said above.
%20(1).png) |
| 21-02-2021 00:19 |
James___★★★★★
(5513) |
Spongy Iris wrote:
I thought ozone was present from approx 6 to 30 miles altitude in the atmosphere, no?
It is. The O2 that starts the Chapman cycle comes from the troposphere. The Chapman cycle is O + O2 + hv > O3 and then O3 - hv > O & O2.
Gasses in troposphere usually move up into the stratosphere near a jet stream.
This is because the jet stream basically borders both the troposphere and the stratosphere. Otherwise the tropopause usually creates a barrier. |
| 21-02-2021 01:01 |
HarveyH55 ★★★★★ ★★★★★
(5196) |
The total mass of ozone in the atmosphere is about 3 billion metric tons. That may seem like a lot, but it is only 0.00006 percent of the atmosphere. The peak concentration of ozone occurs at an altitude of roughly 32 kilometers (20 miles) above the surface of the Earth.Oct 18, 2018
Ozone facts - Nasa Ozone Watchozonewatch.gsfc.nasa.gov › facts
How much ppm of ozone gas is found in the atmosphere?
About 90 % of ozone in the earth's atmosphere is in the stratosphere (12 to 50 km of altitude). It forms a layer where its concentration is higher than anywhere but low ranging from 1 to 20 ppm compared with the oxygen concentration of about 210000 ppm. The ozone layer is what makes the sky blue.
Ozone in the environment - is formed in the atmosphere by ...
Finally got around to Googling it... And CO2 is a trace gas... 20 ppm, max?
Formaldehyde is normally present at low levels (less than 0.03 ppm or 30 ppb) in both indoor and outdoor air. Materials containing formaldehyde can release it as a gas or vapor into the air. Automobile exhaust is a major source of formaldehyde in outdoor air.
30 parts per billion?
I'm really struggling with how these trace gasses can have such a strong influence. |
| 21-02-2021 01:10 |
James___★★★★★
(5513) |
HarveyH55 wrote:
The total mass of ozone in the atmosphere is about 3 billion metric tons. That may seem like a lot, but it is only 0.00006 percent of the atmosphere. The peak concentration of ozone occurs at an altitude of roughly 32 kilometers (20 miles) above the surface of the Earth.Oct 18, 2018
Ozone facts - Nasa Ozone Watchozonewatch.gsfc.nasa.gov › facts
How much ppm of ozone gas is found in the atmosphere?
About 90 % of ozone in the earth's atmosphere is in the stratosphere (12 to 50 km of altitude). It forms a layer where its concentration is higher than anywhere but low ranging from 1 to 20 ppm compared with the oxygen concentration of about 210000 ppm. The ozone layer is what makes the sky blue.
Ozone in the environment - is formed in the atmosphere by ...
Finally got around to Googling it... And CO2 is a trace gas... 20 ppm, max?
Formaldehyde is normally present at low levels (less than 0.03 ppm or 30 ppb) in both indoor and outdoor air. Materials containing formaldehyde can release it as a gas or vapor into the air. Automobile exhaust is a major source of formaldehyde in outdoor air.
30 parts per billion?
I'm really struggling with how these trace gasses can have such a strong influence.
It is no surprise that you're struggling to understand why they matter. I've spent years learning about this and as you said
Finally got around to Googling it...
Just go back over my posts in this thread. I explained the basics.
The basics of why the ozone layer matters;
The ozone layer is a thin part of the Earth's atmosphere that absorbs almost all of the sun's harmful ultraviolet light.
https://www.nationalgeographic.org/encyclopedia/ozone-layer/
Edited on 21-02-2021 01:26 |

%20(1).png)

%20(1).png)
%20(1).png)
%20(1).png)


%20(1).png)
%20(1).png)

%20(1).png)


%20(1).png)
%20(1).png)

%20(1).png)
%20(1).png)