Global warming is caused by ozone depletion, not greenhouse gases
| Global warming is caused by ozone depletion, not greenhouse gases18-11-2014 19:03 | |
| peterlward★☆☆☆☆ (69) |
There is strong evidence that global warming is caused by ozone depletion and that reducing emissions of CO2 and other greenhouse gases is unlikely to have any significant effect on reducing global warming. A press release issued today is attached. Also available at ozonedepletiontheory.info/press-release.pdf The science is explained in detail at ozonedepletiontheory.info I explain the science in a 58-minute talk on YouTube at youtu.be/GyZDf3kMvwo or at tinyurl.com/Peter-L-Ward A summary intended for non-specialists is available at ozonedepletiontheory.info/summary.pdf or from the homepage of the website at ozonedepletiontheory.info A scientific paper is available on the homepage at ozonedepletiontheory.info or at ozonedepletiontheory.info/Papers/Ward2014OzoneDepletionTheory.pdf I have started a Google Discussion Group at the "Science of Ozone Depletion" at https://groups.google.com/forum/?hl=en#!forum/science-of-ozone-depletion While the results are surprising, they are well founded, and, in hindsight, rather obvious. I look forward to your thoughts. Attached file: press-release.pdf |
| 19-11-2014 00:10 | |
| Surface Detail★★★★☆ (1673) |
My first thought is that the author should have carried out a more thorough literature search before putting pen to paper! The climate effects of changing ozone concentrations have already been considered in great detail in a large number of scientific papers. See, for example, N. McFarlane, 2007. Basically, it turns out that reduced absorption of UV radiation in the stratosphere does indeed allow more UV to pass though to the troposphere. However, this results in cooling of the stratosphere, which then gives rise to a substantially larger reduction in the emitted downward IR radiation. The overall effect of this is that stratospheric ozone depletion acts to cool (rather than warm) the troposphere. My second thought is that the author is mistaken in his apparent belief that the temperature of the Earth has been constant for the last 16 years. The simple fact that global sea levels have continued to rise at a steady rate during this period shows that the Earth has continued to warm apace, notwithstanding fluctuations in the distribution of heat between the oceans and the atmosphere. Edited on 19-11-2014 00:14 |
| 19-11-2014 07:29 | |
| peterlward★☆☆☆☆ (69) |
The data clearly show that the stratosphere cools when ozone is depleted. Look at the first plot at ozonedepletiontheory.info/ozone-depletion.html showing ozone levels at Arosa, Switzerland since 1927. The purple line, upper right, shows lower stratospheric temperature anomaly. The references are in the text as well as a discussion of the effects of the Pinatubo, El Chichón, and Agung volcanic eruptions. A widespread misconception is that layers of air in the atmosphere radiate IR downward. This assumes that a layer of gas is a black body. But a black body is defined as a perfect absorber and emitter of radiation. Gas molecules such as CO2, only absorb very narrow frequency bands that are the resonant frequencies of the normal modes of oscillation of the bonds that hold the molecule together. See the third figure at ozonedepletiontheory.info/what-is-radiation.html. This has been studied in great detail. Gas molecules are not perfect absorbers of radiation. Secondly they do not individually radiate energy in the infrared. They can only radiate at higher frequencies when electronic transitions are involved. But this radiation is not constant. A blackbody conducts thermal energy to the surface, where it is radiated. A gas layer does not conduct in the same way. This misconception is, in fact, one of the problems with greenhouse-gas theory. Another problem described on the website and in the talk, is that heat, even radiant heat, flows from a warm body to a cold body. The atmosphere is colder than Earth. Radiant heat just does not flow from cold to warm. That says if you stand next to a cold stove, you will get warmed. Also thermal energy radiated by Earth, does not have high enough frequencies and amplitudes as described in my talk, to warm Earth. A given thermal mass cannot warm itself. Mean global surface temperatures have remained essentially constant since 1998. See the plot of data from the four primary sources at ozonedepletiontheory.info/index.html. Ocean temperature has continued to increase since 1998 because the level of ozone depletion has remained high compared to values before 1970. UV penetrates the ocean tens of meters and is thus absorbed very efficiently by the ocean. This is all described on the same page referring to the second figure and in the talk. |
| 20-11-2014 02:12 | |
| Surface Detail★★★★☆ (1673) |
There is no dispute that the stratosphere cools when ozone is depleted. This is indeed measured and expected, and is entirely consistent with current climate theory. Your claim that there is a mistaken assumption that a layer of gas is a black body is, frankly, bizarre. The entire greenhouse theory is based on the fact that the gases in the atmosphere are not black bodies! If you care to look at it, the scientific literature is full of absorption spectra for different atmospheric gases and the effects of these on energy transfer. Believe it or not, climate scientists are fully aware not only of the absorptive and radiative properties of gases, but also the laws of thermodynamics. The theory you are proposing is naive in the extreme and appears to rely on hand waving arguments (in addition to the intellectually dishonest practice of cherry-picking the year 1998 to make the graphs fit) rather than any calculations or models. In fact, it was determined as long ago as 1976 that reducing stratospheric O3 cools both the atmosphere and the surface, when Ramanathan et al. used a detailed radiative-convective model to determine the sensitivity of surface temperature to O3 concentration. Their work has been validated, extended and refined in numerous scientific papers since then. |
| 20-11-2014 06:06 | |
| peterlward★☆☆☆☆ (69) |
The ten warmest years on record since 1880 are from hottest to coolest: 2010, 2005, 1998, 2013, 2003,2002, 2006, 2009, 2007, and 2004. All four major analyses of mean annual global surface temperature show that 1998 was the beginning of high temperatures for the past 16 years. Greenhouse gas theory posits that infrared radiation from Earth is absorbed by greenhouse gases in the atmosphere, warming those layers of atmosphere, and thereby warming Earth. Warmer gas layers could change the lapse rate so that Earth loses less heat. But the vast majority of heat transfer in the troposphere is by wind and weather. The effect of greenhouse gases on the lapse rate is minimal. Radiation codes used in climate models assume that layers of gas radiate this infrared energy back to Earth similar to black bodies. As you agree, gases are not black bodies. They have minimal thermal mass from which to radiate. While we can discuss the details of radiation ad nauseam, the big picture is that layers of gas in the atmosphere are observed to be colder than Earth. Radiation does not flow from colder to warmer bodies. That violates the laws of thermodynamics. You do not get warm standing next to a colder stove. The bigger issue is that energy in radiation is, according to the Planck postulate, equal to frequency times the Planck constant. Each of us personally can make observations that show this to be true as I explain in the talk. The Planck postulate is not widely understood and differs significantly from the way current radiation codes calculate energy. I explain this in detail in the talk and on the website. Ozone depletion theory is based soundly on clear observations and basic physics. We observe that much UV-B radiation forms, heats, and maintains the ozone layer. We observe and measure that when ozone is depleted, much of this energy reaches Earth. We measure the amount of radiation reaching Earth as a function of total column ozone and its effect on temperature. Those parts of the world that have warmed the most since 1970 are exactly the places where ozone depletion has been greatest. All of this is explained in considerable detail on the website. |
| 20-11-2014 19:19 | |
| peterlward★☆☆☆☆ (69) |
You seem to assume that because I disagree with the prevailing greenhouse-gas theory of global warming, I do not understand climate science and have not read the literature. Au contraire, in the past decade, I have worked my way carefully through more than 10,000 papers on climate science, atmospheric chemistry, atmospheric physics, spectral physics, quantum electrodynamics, and earth science. I have most of these papers available in searchable pdf, the rest in my extensive filing system, and most in my Endnote bibliographic database. I have a very complete library of climate textbooks and key reports on climate since 1800. And being retired, I have been able to focus the vast majority of my time and energy on climate science, seven days a week for a decade. I understand current climate science very clearly. I also have more than 50 years' experience as a scientist, with several years as leader of a major US National research program, associate editor of a highly respected journal, member of a working group assembled by then Vice President Al Gore, and Chair of a working group at the White House Office of Science and Technology. What I have done that is different from most climate scientists, is to try to understand in precise detail what thermal energy is and how it is transferred through air and space from one body of matter to another. I explain this in the talk and on the website. This line of research has proven to me beyond any reasonable doubt, that greenhouse-gas theory, as implemented in radiation codes at the heart of all climate models today, is physically impossible. I have spent over a year writing a book-length website documenting the details in a fully referenced manner. I have spent months developing a scientific talk that summarizes the details as cogently as I can at the moment. I have written a scientific paper to be published soon by CRC Press explaining some of the details and available now on the website. I have written numerous scientific papers over the past decade, most of which have been rejected without review because they disagree with greenhouse-gas theory. All of these papers and the comments received are on the ozonedepletiontheory.info website. The facts are quite clear. Greenhouse-gas theory cannot explain observed global warming and ozone depletion theory explains the wide range of observations not only in the last 100 years but throughout geologic time in much more detail and much more clearly than greenhouse-gas theory. The greatest warming in the earth system is maintained in the stratosphere. This warming of up to ~70 degrees C, is caused and maintained by the ongoing photo-dissociation of O2, O3, and many other chemical species, absorbing the highest-energy ultraviolet radiation penetrating through the ionosphere and mesosphere. When you understand that radiant energy is a function of frequency, as can be observed very clearly, it becomes clear that the temperature of Earth's surface is determined primarily by the highest energy solar radiation penetrating the atmosphere and that the changes in the temperature of Earth's surface are determined primarily by the changes in the highest energy solar radiation penetrating the atmosphere. I look forward to discussion with any scientist who is willing to objectively evaluate the data and logic. We do not have to agree, but we do need to do the hard work of science. With the world arguing about spending major amounts of money to reduce greenhouse gases as urged by scientists, scientists have a major responsibility to be sure that their theories are up to date and based on the best scientific data and logic available. |
| 23-11-2014 05:41 | |
| Abraham3 (256) |
You are Peter Ward, the vulcanologist? https://en.wikipedia.org/wiki/Peter_Langdon_Ward |
| 23-11-2014 15:38 | |
| peterlward★☆☆☆☆ (69) |
Yes, although the Wikipedia page is many years out of date and geophysicist would be a more inclusive descriptor than volcanologist. In 2006, I found on the World Wide Web the excellent GISP2 data showing unambiguously that sulfate in Greenland ice reached its highest rates of deposition just as the world was warming out of the last ice age. Thus increased volcanism was contemporaneous with global warming. This did not make sense because volcanologists and climatologists know that all major explosive volcanic eruptions in history were followed by global cooling of about 0.5oC for typically three years. I recognized that understanding this enigma might lead to important science and decided to put aside most other things in my life to concentrate on this issue (ozonedepletiontheory.info/three-enigmas.html). The first possibility to evaluate was that increased SO2 caused increased warming just as most climatologists think increased CO2 concentrations coming out of ice ages is one proof that greenhouse gases cause global warming. About the time my 2009 paper came into print, I realized that SO2 is the footprint of volcanism, that volcanism causes ozone depletion, and that ozone depletion causes warming. In 1993, following the eruptions of Pinatubo and Cerro Hudson in mid and late 1991, total column ozone at mid-latitudes reached its lowest values since measurements began in 1927 (ozonedepletiontheory.info/ozone-depletion.html). It took considerable work to understand that both effusive and explosive volcanic eruptions deplete ozone, warming the world, but that explosive eruptions also form aerosols in the lower stratosphere that reflect and scatter sunlight, causing net global cooling (ozonedepletiontheory.info/volcanoes-and-climate.html). This is where my background in volcanology paid off. I began to wonder about the precise physics and chemistry of global warming and spent years coming up to speed in these fields. I found that no physicist had seriously questioned greenhouse-gas theory since the work of Knut Ångström in 1900 (ozonedepletiontheory.info/Papers/Angstrom1900-English.pdf), when he showed in the lab and in the field, that absorption of infrared radiation by CO2 did not seem to have much effect on the temperature of air. I began to question the assumptions made in all radiation computer codes used in climate models. I can now describe in considerable detail on the website and in the YouTube talk why greenhouse-gas theory as widely visualized is not physically possible. It just is not the way thermal energy is emitted and absorbed in the atmosphere. Ozone depletion theory, on the other hand, provides a very clear explanation for global warming that fits all the data on thermal energy observed penetrating Earth's atmosphere and can be tested in many ways. As I point out in the YouTube talk, if greenhouse gases actually cause atmospheric warming, it should be possible to measure this in the laboratory. Ångström's paper is the only one in the literature that I can find that actually does the experiment, and he concludes that increases in CO2 do not cause any significant warming. As a result of this paper, physicists lost interest in greenhouse-gas theory. But the discredited greenhouse-gas theory was resurrected 38 years later by a steam engineer and ultimately by geochemists (ozonedepletiontheory.info/gg-comments-on-history.html). |
| 24-11-2014 05:24 | |
| Abraham3 (256) |
peterlward wrote: Was there any other evidence of increased vulcanism during that time period? How long was this period of increase sulfate deposition? Did you find high levels of sulfate deposition at the exit of other glaciations? Did these correlate with known volcanic events or active periods? Did known large volcanic events: the creation of the Deccan Traps or the Columbia Shield coincide with evidence of warming? peterlward wrote: You are correct of course about the short term cooling from sulfate aerosols in the atmosphere. Thus I am having a problem understanding how such an impulse could be the driver behind a process - exiting a period of glaciation that takes thousands and thousands of years. peterlward wrote: I think you would have a very hard time finding a climatologist that did not believe increase atmospheric CO2 will cause increased warming, but the increase in CO2 in the atmosphere when temperatures increase is not the greenhouse effect in action. It is the reduction in the ocean's carbonate solubility from increased temperatures. As Jeremy Shakun and others have found, warming from that CO2 can eventually overwhelm the Milankovitch warming, but it is not the root cause of the glaciation exit. peterlward wrote: So as your 2009 paper came out you realized that it was incorrect? That it was not sulfates from fossil fuel combustion but from volcanoes? And those sulfates were not increasing the lifetime of water vapor and other greenhouse gases, but depleting ozone. Your 2009 paper stated that global warming was defeated by efforts in the US and Europe to reduce SO2 emissions to combat acid rain. You claimed that was sufficient to eliminate global warming worldwide by the end of the century. Now you claim the source of that SO2 was vulcanism, not fossil fuels. You really don't seem to have a coherent picture going here. peterlward wrote: Good grief. You sound as if you're rejecting the greenhouse effect based on the work of Angstrom. I truly hope that is not the case. Please explain. |
| 24-11-2014 18:41 | |
| peterlward★☆☆☆☆ (69) |
These are all very thoughtful questions. The answers are mostly explained in detail on the website ozonedepletionheory.info. The reason for creating this website was to make the technical details readily available to anyone interested. The website is the electronic equivalent of a rather large book divided into 28 chapters (menu items) with more than 70 illustrations and hundreds of references with hyperlinks to the actual papers. The 58-minute video at tinyurl.com/Peter-L-Ward is meant as an overview and introduction. There is also a 6-page, less technical summary for non-specialists at ozonedepletiontheory.info/summary.pdf. But both an hour talk and a book can be intimidating to start, so here are shorter answers. The younger parts of Iceland are covered with tuya or table mountains that are formed by primarily basaltic volcanoes erupting under ice. "12 of the 13 dated table mountains experienced their final eruptive phase during the last glaciation" (Licciardi et al., 2007) dominantly between 11,750 to 9,375 years ago when the rate and continuity of sulfate deposition were both high and the most rapid warming occurred as shown by the δ18O proxy for temperature measured in the same ice. Sulfate also increases rapidly at the beginning of the 25 Dansgaard-Oeschger rapid warmings over the past 120,000 years, although the signal to noise ratio decreases for data older than about 46,000 BP. This is described and plotted at ozonedepletiontheory.info/abrupt-climate-warming.html and in more detail in my 2009 paper. Some volcanic complexes in Iceland appear to be dated at these older times of rapid warmings but there are insufficient data to be absolutely sure. Essentially all major mass extinctions and periods of major warming and acidic oceans in the past 360 million years are contemporaneous with major continental flood basalts (Courtillot and Renne, 2003). Other periods of rapid warming throughout geologic time, such as the Paleocene-Eocene Thermal Maximum (Storey et al., 2007), are contemporaneous with major increases in basaltic volcanism. See ozonedepletiontheory.info/volcanoes-and-climate.html and the video. One of my most important contributions is recognition of the fundamental difference in climate effects of effusive basaltic volcanoes that cause warming and explosive volcanoes that cause cooling. All volcanism causes ozone depletion and warming. Explosive volcanoes, however, eject megatons of SO2 and water into the lower stratosphere forming sulfuric-acid aerosols that reflect and scatter solar radiation leading to net cooling. I argue that climate change throughout geologic time was driven by which of these types of volcanism was dominant at the time, which was determined by the configuration of the plates making up the surface of the earth. See ozonedepletiontheory.info/volcanoes-and-climate.html, ozonedepletiontheory.info/summary.pdf, and especially the video. One can argue that the changes in CO2 concentrations going into and out of ice ages over the past million years is simply a proxy for ocean temperature because of the effects of ocean temperature on solubility. Most detailed studies show that increases in CO2 lag behind increases in temperature. See references at ozonedepletiontheory.info/climate-trends-and-depletion.html. Milankovitch cycles appear to have some effect on ice ages, but the correlation coefficient is not very high. I argue that glacial periods are controlled primarily by volcanism. Milankovitch cycles cannot explain the sudden onset and demise of glaciation that is well observed throughout the geologic record. In the 2009 paper I show that SO2 increases from volcanism are typically contemporaneous with rapid warming and that SO2 emissions by humans burning fossil fuels in the 20th century appeared contemporaneous with recent warming, suggesting both may cause the same mechanism for warming. These observations are well documented and are still accurate. What has changed is the realization that SO2 is merely the footprint of volcanism and fossil fuel burning. In the case of volcanoes, the eruptions deplete ozone, causing the warming. In the case of fossil fuels, the time lag between increased SO2 and increased temperature appears too long to be a cause of the warming. See [url]ozonedepletiontheory.info/ climate-trends-and-depletion.html[/url]. Warming since 1945 now appears directly related to the emission of chlorofluorocarbons and the resulting ozone depletion. The time lags in this case are readily explained. See ozonedepletiontheory.info/climate-trends-and-depletion.html, ozonedepletiontheory.info/summary.pdf, and especially the video (tinyurl.com/Peter-L-Ward). I reject the greenhouse-gas theory of climate change because as formulated and modelled, it is physically impossible. This is explained most cogently in the video (tinyurl.com/Peter-L-Ward), the summary (ozonedepletiontheory.info/summary.pdf), and at ozonedepletiontheory.info/primary-problem-with-GG.html. The basic issues are what is thermal energy and how is it transferred through air and space? Thermal energy is assumed by greenhouse-gas theory to be a function of wave amplitude and effectively of bandwidth. But waves travel by deforming the bonds in matter. There is no matter in space and there are no bonds. In space, energy is equal to the frequency of the radiation times a constant. See Planck postulate in Wikipedia. We all observe this reality. Nuclear radiation is very high frequency and very high energy. Ultraviolet-B radiation has enough energy to burn your skin. Visible light has enough energy to drive photosynthesis, the growth of plants. Infrared radiation does not have enough energy to burn skin or drive photosynthesis. In fact, the energy in ultraviolet radiation reaching Earth when ozone is depleted is 48 times greater, 48 times hotter, than the energy of infrared radiation absorbed by greenhouse gases. A second problem: Planck's law shows that to warm a body of matter, you must increase the amplitude of oscillation of the molecular bonds at every frequency and you must increase the frequency of the peak amplitude. This means that a body of matter cannot be warmed by radiation from the same body or from a colder body. Infrared radiation emitted by Earth, cannot warm Earth no matter how efficiently you radiate it back to Earth. A third problem: We know temperatures decrease going up in the atmosphere, the lapse rate. Therefore any layer of gas in the atmosphere is colder than Earth. You do not stand next to a cold stove to get warm. Heat does not flow that way. Fourth: layers of gas are modeled in the radiation codes as radiating in a matter similar to black bodies. Gas is not a black body and it does not have the thermal mass to radiate. The surface of matter radiates because thermal energy is conducted in to replace the thermal energy radiated. This is all explained carefully in the video (tinyurl.com/Peter-L-Ward), in the summary (ozonedepletiontheory.info/summary.pdf), and on the website (ozonedepletiontheory.info). It is not easy to understand at first because these problems are rooted in a basic misunderstanding in physics since the 1860s as explained in the video. It took me a couple of years to become really comfortable with it and to reluctantly reach the conclusion that there was a fundamental problem in basic physics. Planck's postulate led to modern quantum physics and he got the Nobel Prize for it. But even Planck did not fully realize the implications of this postulate. The best introduction to this is the video, which I produced most recently. I am working on a paper describing it in more detail. Thank you for the questions. I hope these answers help you to delve more deeply. |
| 25-11-2014 13:55 | |
| Abraham3 (256) |
A thousand pardons for not watching your video or reading your book. I can explain the greenhouse theory and AGW in text within a few paragraphs. I cannot help but feel that if your theory cannot be explained under such constraints it has a problem. I am not in the least surprised that there is a correlation between large scale vulcanism and major extinction events in the Earth's history. Sulfate aerosols cause cooling. The increase in effective albedo they create is spectrum-wide. An decrease in ozone is quite spectrum-specific. I have a very difficult time believing that the latter would outweigh the former.
You are, of course, aware that an extremely high proportion of your fellow physicists and scientists of every other sort, disagree with you completely.
So, you are contending that thermal energy is not quantified by wave amplitude and (frequency and) bandwidth ? And you believe that electromagnetic radiation physically deforms the matter through which it travels? If I didn't understand you to be a trained physicist I might guess that you had confused EM waves with vibrations.
I have to get to work and don't have time to respond as I would but let me give you two scenarios to consider. I have a mass with an integrated heating element. The heating element is being fed electrical current from outside the system. The mass is immersed in a heat sink. The mass will eventually attain thermal equilibrium. What happens to its temperature if I enclose the mass in a layer of insulation? and I am sitting in front of my living room fireplace with a fire going. Again, I have reached thermal equilibrium. Suddenly the walls and roof of my house disappear and I am now surrounded by snow-covered plains to the horizon and the starry night sky above. What happens to my temperature? In both cases, my surroundings were colder than my 93F body. If my 72 degree walls can't radiate to me, why the difference? |
| 25-11-2014 17:45 | |
| peterlward★☆☆☆☆ (69) |
What is wrong with greenhouse-gas theory can be stated very simply in one small equation, the Planck postulate (E=hν): the thermal energy in radiation (E) is equal to the frequency (ν, Greek letter nu) times the Planck constant (h) and has nothing to do with amplitude, wavelength, or bandwidth. The problem is that you and most others have not thought much about the Planck postulate and it takes some effort to understand because it is so different from the way we are used to thinking. Even Planck did not fully comprehend it at the time, although he got the Nobel Prize for it because it led to quantum mechanics. E=hν is used widely in photochemistry to designate the thermal (chemical) energy required to cause a chemical reaction such as the photodissociation of N2, O2 or O3. We all observe quite clearly that nuclear radiation has more energy than X-rays, that have more energy than ultraviolet radiation, that has more energy than visible light, that has more energy than infrared radiation. X-rays are used to kill cancer cells. Ultraviolet-B radiation burns your skin. Visible light powers photosynthesis. All the infrared radiation in the Universe cannot power photosynthesis, burn your skin, or destroy cancer cells. Thermal (chemical) energy is a function of frequency, NOT amount, amplitude or bandwidth. There is the issue of dosage where it takes a minimum amount of radiation to affect DNA, for example, within a certain unit of time. Low dosage of X-rays allow us utilize their energy high-enough to penetrate your body and expose film while keeping the dosage so low that they do not damage your body. High dosage of X-rays is used to kill cancer cells. Lookup up the Electromagnetic spectrum part of the Wikipedia page for electromagnetic radiation. Physically, thermal energy IS frequency. When you heat a body of matter, the frequency and amplitude of the oscillations of the bonds holding matter together are measured to increase. This is shown most clearly by Planck's law for radiation from the surface of a body of mass at thermal equilibrium. Planck's law was derived empirically to explain the observed physical properties of thermal radiation. Frequency and amplitude are clearly observed and measured, although we think of amplitude as spectral radiance, which has some historic issues that I describe on the website. We derive wavelength mathematically by dividing what we call the velocity of light by the frequency. Units wise, this is meters per second over cycles per second, which is meters per cycle. But if frequency of radiation (cycles per second) is equal to energy (E) divided by the Planck constant (h), then what we call wavelength has units of meters per second divided by a function of Energy where the Planck constant is the energy contained in the frequency of one cycle per second. We do not physically observe wavelength. It is a mathematical property not a physical property. We do observe frequency. Frequency is color. Every shade of color has a distinct frequency. White light has a broad range of frequencies (colors). Everything we see is frequency that causes the rods and cones in our eyes to resonate, sending neurological signals to our brain that we interpret as color. Every molecule out there that we observe radiates color (frequency) that causes these resonant oscillations in our eyes. This is what Einstein called "Spooky action at a distance": a physical property of something over here causes a change in the physical properties of something over there and we do not see any connection between them. This connection is via frequency and this is quantum entanglement. It is the way we see the world. It is also the way we hear the world around us. Frequencies cause resonance in the hair cells within our ears. Now frequency in radiation does cause phenomena when interacting with matter that we find easiest to explain in terms of waves such as reflection, birefringence, the splitting of light by a prism or the water molecules causing a rainbow, or the interference seen in the classic double-slit experiments. Frequencies of light do not interfere with each other in space but they do when interacting with matter. There is a long and rich history of the wavelength view of radiation especially since the work of Maxwell in the 1860s and this approach underlies greenhouse-gas theory. But radiation, light, thermal energy in space is frequency and is not wavelength. In the latter half of the 19th century, numerous scientists proved that there was no luminiferous aether through which light waves travelled. Look up "luminiferous aether" and "timeline of luminiferous aether" in Wikipedia. The thermal energy of ultraviolet-B radiation that reaches Earth when ozone is depleted is, according to E=hν, 48 times more energetic, 48 times hotter that the infrared energy absorbed by greenhouse gases. If you think of the infrared as a candle, ultraviolet-B is a very hot blowtorch. |
| 25-11-2014 19:37 | |
| Surface Detail★★★★☆ (1673) |
E=hν is the Planck-Einstein relation (not the Planck postulate), and it simply expresses the energy E carried by an individual photon of light of frequency ν. It is not a measure of the total radiant power, which of course depends on the number of photons as well as their individual energies. It is perfectly possible to have UV and IR fluxes of the same intensity, it's just that the UV flux has fewer, high-energy photons, while the IR flux has more, lower-energy photons. The Planck postulate, E=nhν, where n is an integer, expresses Planck's assumption that the energies of the photons emitted by a black body can only take on certain values, i.e. they are quantised. Although he wasn't able to justify the assumption (that came later), it allowed him to derive a formula that accurately reflected the spectrum of the radiation emitted by a black body. |
| 25-11-2014 21:58 | |
| Abraham3 (256) |
Thanks. |
| 25-11-2014 23:19 | |
| peterlward★☆☆☆☆ (69) |
Planck describes quite clearly how in 1900 he had to postulate that E=hν in order to write the equation for radiance from a black body at a given temperature that became known as Planck's Law. See the formulation ozonedepletiontheory.info/ImagePages/Plancks-law-math.html. In 1905, Einstein picked up on it in describing the photoelectric effect, calling hν a light quantum. Ultimately the quantum mechanics way of expressing it was nhν. It was Lewis in 1926 that came up with the word photon. You are correct that E=hν is known as the Planck-Einstein relation but Planck did not know of Einstein until 1905 when Einstein submitted his papers to Annalen der Physik of which Planck was editor. While it is commonly assumed that you can have UV and IR fluxes of the same intensity, you clearly cannot if intensity means energy. You can have IR and UV fluxes of the same brightness, the same amplitude, but not the same energy. UV-B flux will burn your skin. All the IR flux in the Universe cannot burn your skin. This is quite clear, but not widely understood. Several years ago I tried to find out what happens physically when a photon interacts with a molecule of CO2. Spectral physicists study in great detail how molecules such as CO2 absorb energy from an electromagnetic field. Absorption is along narrow spectral lines and these lines turn out to be the frequencies of all the normal modes of oscillation of all the different modes of oscillation of the bonds that hold the molecule together. These spectral lines are tabulated in the HITRAN database in great detail for most common gases and are used to determine the composition of distant atmospheres and things near at hand because each gas has very distinctive spectral lines unique to that gas. If we think of the photon as the packet of energy transferred, then the details of this packet, the many different characteristic frequencies, are determined by the receiver, the molecule, not by the source of the photon at the sun. This says the photon was created by the molecule at the molecule from the energy field. The photon is a very handy shorthand for the energy transferred and is used widely, especially in quantum physics, but it is a mathematical construct, not a physical reality. There are many other arguments for why a photon is not a physical thing if we need to go there. Again, look at the table wikipedia.org/wiki/Electromagnetic_radiation#mediaviewer/File:Light_spectrum.svg to see the relationship between frequency and energy in electromagnetic radiation. This table also includes wavelength as I described before. Or look at ozonedepletiontheory.info/ImagePages/EM-spectrum-properties.html. Energy and temperature are closely related to frequency. These are things we observe. They are not theoretical. And this issue at hand is how does thermal energy warm the Earth? |
| 26-11-2014 00:02 | |
| Abraham3 (256) |
Planck–Einstein relation From Wikipedia, the free encyclopedia (Redirected from Planck-Einstein relation) The Planck–Einstein relation,[1][2] also referred to as the Einstein relation,[1][3][4] Planck's energy–frequency relation,[5] the Planck relation,[6] and the Planck equation,[7] is a formula integral to quantum mechanics, and states that the energy of a photon (E) is proportional to its frequency (ν). 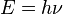 The constant of proportionality, h, is known as the Planck constant. Several equivalent forms of the relation exist. The relation accounts for quantized nature of light, and plays a key role in understand phenomena such as the photoelectric effect, and Planck's law of black body radiation. See also the Planck postulate. Edited on 26-11-2014 00:05 |
| 26-11-2014 00:39 | |
| peterlward★☆☆☆☆ (69) |
Yes we all agree that this is the standard definition. But in my previous post I gave the logic for why a photon is not a physical reality and I can give more logic if you want. What is clearly observed in the laboratory is that the distribution of frequency (energy) in a photon is set by the physical parameters of the receiver. We also observe in nature that E=hv applies to all electromagnetic radiation and that infrared radiation never contains enough energy to burn your skin or photodissociate oxygen or cause photosynthesis. The issue at hand is what is radiant thermal energy and how does it differ from the ultraviolet to the infrared. The concept of a photon is deeply ingrained in our thinking. It is in the standard model of physics. But one of the major problems with quantum mechanics is that it does not make physical sense. The first thing you are taught in quantum mechanics is don't sweat it. It is not intuitive. As is attributed to Richard Feynman "Just shut up and calculate". I believe that physics has to be physical and have spent a lot of time trying to understand it based solely on physical observations. E=hv can be clearly observed as it applies to all electromagnetic radiation. If you are only willing to think inside the box, you can defend photons to your death, but you will miss out on what is actually physically happening. One reason my website is so large, is that I report a wide range of observations that demonstrate the ozone depletion theory. The greatest warming is where ozone has been depleted the most. This explains Arctic Amplification, something clearly observed that greenhouse-gas theory has no explanation for. Etc. |
| 26-11-2014 01:24 | |
| Surface Detail★★★★☆ (1673) |
peterlward wrote: Now that is just a bizarre thing to say. Stand too close to a hot fire, and the IR flux from the fire will heat your skin and burn it. Granted, it's a different sort of burn to a UV burn ("sunburn") in that it results from heating of the tissues rather then DNA damage, but it's still a burn! |
| 26-11-2014 04:22 | |
| peterlward★☆☆☆☆ (69) |
So what do you mean by a "hot fire"? If you can see a hot fire, it is not radiating just IR. The peak frequencies radiated are in the visible spectrum. White hot radiates all visible frequencies. Red hot means the peak frequencies are only in the red visible spectrum. Planck's law tells us what the radiation is from a body as a function of temperature of the surface of the body. The frequency with the peak amplitude on a Planck curve is calculated from the Wien Displacement Law as 5.879x10^10 times temperature. For terrestrial radiation, T = ~288K, frequency =~17 Thz, energy= 0.07 electronvolts. That radiation will not burn your skin, no matter how much of it exists. For boiling water with T= 373K, max frequency =~23 Thz, energy 0.09 eV. The radiation from boiling water will not burn you but the steam will. The filament of an incandescent light bulb has a temperature around 3,300K, frequency around 200 Thz, energy around 0.8 eV. The radiation from a light bulb will not burn you, put touching the light bulb will and touching the filament would be pretty nasty. Visible light from the sun comes from a black body temperature around 5570K with frequencies from 400 to 790 Thz and energies from 1.6 to 3.26 eV. Visible light will not burn your skin. UV-B has frequencies around 1000 Thz, energy around 4 eV and a blackbody peak temperature around 17,000K. You have to be careful sorting out heat transfer by radiation versus by conduction. |
| 26-11-2014 05:12 | |
| Abraham3 (256) |
You would seem to have stood before few fires. I guarantee you they can burn you with infrared. |
| 26-11-2014 05:31 | |
| peterlward★☆☆☆☆ (69) |
As I said, if you can see the fire, the main radiation is in the visible not in the infrared. As an outdoorsman, I have sat around many fires, including huge bonfires traditional at Dartmouth before big football games. You can get very warm and the air circulating past you can be very hot. But the radiation leaving the fire does not burn you. You get out of the way before that can happen because you are so warm. And it still cannot happen until the radiation gets much hotter. If you touch the fire or a hot rock, you can get burned by conduction just as when you touch a hot frying pan. Visible light comes from very hot sources and yet it does not burn your skin until in the ultraviolet. Infrared radiation cannot burn your skin. Google "erythemal radiation" or check out http://www.iac.ethz.ch/en/research/chemie/tpeter/www_uv.html for the response function of your skin to sunburn. |
| 26-11-2014 11:23 | |
| Surface Detail★★★★☆ (1673) |
No, that is wrong. The vast majority of the energy radiated by, say, a bonfire is in the IR part of the spectrum. See, for example, http://en.wikipedia.org/wiki/Flame_detection#Emission_of_radiation. Only a very small fraction of the energy is radiated in the visible and UV. Also, most of the heat you feel when standing by a bonfire is indeed radiated heat. Air is not a good conductor of heat; if it were, then you wouldn't need to turn round to warm your back when standing near a camp fire! It's not convection either, since the air flow is typically towards the fire as cold air is sucked in to fill the void left by the hot air rising from the fire. |
| 26-11-2014 12:54 | |
| Abraham3 (256) |
peterlward wrote: That is complete nonsense. You can be burned by ANY radiation that your skin absorbs and your skin most definitely absorbs IR. |
| 26-11-2014 15:54 | |
| peterlward★☆☆☆☆ (69) |
Erythemal (meaning sunburning) radiation is studied in great detail for health reasons. The erythemal action spectrum is shown and discussed quite clearly at http://www.iac.ethz.ch/en/research/chemie/tpeter/www_uv.html and many other places including https://ozonedepletiontheory.info/primary-cause-of-warming.html with reference to Gerstl, 1981 at http://dx.doi.org/10.1038/294352a0 title="Biologically damaging radiation amplified by ozone depletions". Again, the radiation that gets you really hot is not simple IR. IR absorbed by greenhouse gases is only as hot as ~15oC. Radiation from a white hot fire is primarily in the visible spectrum. The spectral makeup of radiation is easily measured and that is what Planck's law is all about. Much of what you perceive as hot is convection, not radiation. |
| 26-11-2014 18:14 | |
| Surface Detail★★★★☆ (1673) |
Nobody is disputing the damaging effects of UV radiation on skin; nor is anybody disputing the ability of ozone to block UV radiation. I think we're all on the same page there! What we do dispute is your peculiar claim that you cannot be burned by infra-red radiation. As you quite correctly state, "the spectral makeup of radiation is easily measured and that is what Planck's law is all about". Plugging the numbers into Planck's law tells us that a wood fire at 1500° K puts out peak radiation at about 2000nm, with 98% of its radiation beyond 1000nm. As I said, only a very small fraction of the energy of the energy from a camp fire is radiated in the visible and UV; it's almost all IR, which can indeed burn you! |
| 26-11-2014 18:40 | |
| peterlward★☆☆☆☆ (69) |
Please read http://en.wikipedia.org/wiki/Burn#Radiation and http://en.wikipedia.org/wiki/Radiation_burn. This is well understood and while it might not seem to agree with your perception, you have to distinguish between burn caused by radiation versus burns caused by scalding, contact, etc. Radiation that burns skin must be at least as energetic as ultraviolet. Most of the damage occurs for wavelengths less than 320 nanometers, frequencies greater than 936 terahertz, energies greater than 3.9 electronvolts. See the plot and references at https://ozonedepletiontheory.info/ImagePages/erythemal-relative-response.html. |
| 26-11-2014 19:09 | |
| Surface Detail★★★★☆ (1673) |
From he second of your links:A radiation burn is damage to the skin or other biological tissue caused by exposure to radiation. The radiation types of greatest concern are thermal radiation, radio frequency energy, ultraviolet light and ionizing radiation. Thermal radiation includes IR radiation, and radio frequencies are far lower than UV frequencies. And then there are microwaves. As Abraham3 stated, any radiation absorbed in sufficient quantity by the skin will burn it, albeit in different ways. There are numerous references on the internet to the possibility of sustaining burns from IR radiation. You can even buy so-called "heat lamps" that are optimised to radiate in the IR part of the spectrum and are frequently used to provide outdoor heating during the winter here in the UK! |
| 26-11-2014 19:24 | |
| peterlward★☆☆☆☆ (69) |
Sorry but you are wrong. Read the references I sent. To burn skin, to cause it to turn red, with only radiation, the radiation must be hot enough at the levels determined by detailed scientific studies summarized in the relative response curves in those references. Now you can heat things and cook things with microwaves but this is very different physics and biology from sunburn. Wikipedia has good articles on microwaves and microwave ovens and how they work. Please read the science. It is pretty clear. |
| 26-11-2014 19:54 | |
| Surface Detail★★★★☆ (1673) |
The science certainly seems clear enough to me. As, presumably, it does to the people who make infrared heaters. Sit too close to one of these for too long, and the side of you facing the heater will burn! |
| 26-11-2014 23:04 | |
| Abraham3 (256) |
Mr Ward, When Surface Detail and I use the term "burn" we are using one of these: to undergo rapid combustion or consume fuel in such a way as to give off heat, gases, and, usually, light; be on fire: The fire burned in the grate. 2. (of a fireplace, furnace, etc.) to contain a fire. 3. to feel heat or a physiologically similar sensation; feel pain from or as if from a fire: The wound burned and throbbed. 4. to give off light or to glow brightly: The lights in the house burned all night. 5. to give off heat or be hot: The pavement burned in the noon sun. 6. to produce pain or a stinging sensation similar to that of fire; cause to smart: The whiskey burned in his throat. We are NOT intending SUNBURN. Yet that is what you're falling back on. I believe your point is lost. Humans may be BURNED by any radiation their bodies will absorb. There is no limit to the amount of energy any particular EM frequency may carry. That amount is most certainly dependent, among other things, on the amplitude of the EM radiation. |
| 26-11-2014 23:57 | |
| peterlward★☆☆☆☆ (69) |
My initial statement was "Infrared radiation does not have enough energy to burn skin or drive photosynthesis." By burn skin I mean to have the effects explained at http://en.wikipedia.org/wiki/Sunburn. Sunburn is well studied and measurements in the lab and field show that initial sunburning is caused by ultraviolet radiation with wavelengths <400 nanometers and becomes serious for wavelength <325 nm. See http://ec.europa.eu/health/scientific_committees/opinions_layman/en/sunbeds/figtableboxes/figure-2.htm for the CIE (1987) reference action spectrum for erythema in human skin. If you want to define burn as "give off heat or be hot", then obviously infrared radiation does that. But that is irrelevant to my initial statement that we were discussing. The nearest infrared, the hottest or most energetic infrared has frequencies less than 400 Terahertz and energies less than 1.6 electronvolts. Sunburning ultraviolet radiation at 325 nm, has frequencies >922 Thz and energies >3.8 electronvolts. Again "infrared radiation does not have enough energy to burn human skin." |
| 27-11-2014 00:02 | |
| Abraham3 (256) |
I thought we were discussing global warming. IR's effect on skin has no bearing. That IR can heat any substance which absorbs it is a crucial point. What relevance do you believe human sunburn has to global warming? |
| 27-11-2014 04:42 | |
| peterlward★☆☆☆☆ (69) |
I have made the point quite clearly that for radiation in space, energy is equal to frequency times the Planck constant. I have stated that we can see directly that this relationship is true by seeing that infrared radiation does not have enough energy to cause photosynthesis or sunburn. Visible light has enough energy to cause photosynthesis. Ultraviolet light has enough energy to cause sunburn. I give a much more extended description of this in the video. When we accept that E=hv, we see that the ultraviolet energy that reaches Earth when ozone is depleted is at least 48 times hotter, 48 times more energetic than the infrared energy absorbed by greenhouse gases. |
| 27-11-2014 13:23 | |
| Abraham3 (256) |
I'm Joe astronaut out on the ISS. I have a 25 mW UV flashlight and a 1 GW UV laser (secret weapons research ;-)). They emit the exact same frequency. Are you saying that when I shine them both out the port, they will have equal energy? |
| 27-11-2014 16:07 | |
| peterlward★☆☆☆☆ (69) |
Yes, that is the main point. Energy of radiation in space is directly proportional to frequency and is not a function of the amount of energy. The amount of energy, the brightness, the amplitude are a function of the power of the source and the inverse square of the distance radiated. A red light has a spectrum of frequencies that do not interact or change even over galactic distances. Each frequency has an energy capable of causing some chemical reaction. The reason for this is that there is no matter in space and there are no bonds holding matter together in space. Interaction of frequencies in matter occurs because of the molecular bonds. I explain this in some detail in the video. This is perhaps the hardest concept to understand because it is not what we are used to thinking. However, the evidence for it is very strong. |
| 27-11-2014 16:13 | |
| Abraham3 (256) |
Energy is not a function of the amount of energy? Really? As Surface Detail noted, you are confusing a single photon with radiation. The energy content of a single photon is proportional to its frequency. It has no other variable parameter. The amount of radiation in a system is dependent on the frequency AND NUMBER of photons. I guarantee my astronaut's GW laser is producing more UV photons than is his UV flashlight and thus is transmitting more energy to whatever it is at which the two of them are aimed. Edited on 27-11-2014 16:17 |
| 27-11-2014 16:42 | |
| peterlward★☆☆☆☆ (69) |
We are going in circles. I have explained to you that radiation in space is not photons and is not waves. It is frequency. This is not commonly accepted yet, but the evidence for it is very clear. Please watch the video. It will save you a lot of time and energy. You still may not believe me, but you will at least understand the science a little better. Real science takes work and careful thought. And this is right at the crux of why greenhouse-gas theory is misguided. Understanding it is not going to come easily because it is not what most people have been thinking. And the observational evidence for why greenhouse-gas theory may be misguided is laid out meticulously on the website. |
| 27-11-2014 17:00 | |
| Abraham3 (256) |
Sorry, but I do not believe you. |
| 27-11-2014 17:59 | |
| peterlward★☆☆☆☆ (69) |
Science is about observation and analysis, not belief. You will never believe me if you do not consider, evaluate, and analyze what I have gone to great pains to explain in an easily approachable video and in a detailed website. Are you open to learning? |
| 27-11-2014 20:59 | |
| Surface Detail★★★★☆ (1673) |
peterlward wrote: Sorry, but this just sounds like new-age pseudoscience to me. Radiation cannot be frequency. Frequency is simply the number of occurrences of a repeating event, such as a wave, per unit time. It is very well established by detailed theory and countless experiments that electromagnetic radiation has both wavelike and particulate characteristics, and that the energy carried by each "particle" of radiation (i.e. photon) is proportional to the frequency of that radiation. This theory of radiation underpins pretty much the whole of modern physics and is explained in school-level physics textbooks. The probability that you are, somehow, right and the entirety of modern physics is wrong is vanishingly small. Edited on 27-11-2014 21:00 |
Join the debate Global warming is caused by ozone depletion, not greenhouse gases:
Related content
| Threads | Replies | Last post |
| The "radiative Greenhouse effect" does not exist | 141 | 22-04-2024 21:05 |
| 'Greenhouse' Effect? | 49 | 30-11-2023 06:45 |
| The SCIENCE of the "Greenhouse Effect" | 291 | 05-11-2023 22:46 |
| How covid 19 vaccine caused Jamie Foxx to become blind and paralyzed | 2 | 08-06-2023 03:23 |
| Nitrate Reduction - Powerful Greenhouse Gas Emission AND Alkalinity | 102 | 05-06-2023 13:19 |