Climate Change - Vicious Feedbacks and Worst-Case Scenarios
| 24-03-2022 14:41 | |
| Tim the plumber★★★★☆ (1356) |
sealover wrote: Only significant impact of increased CO2 so far; Attached image: 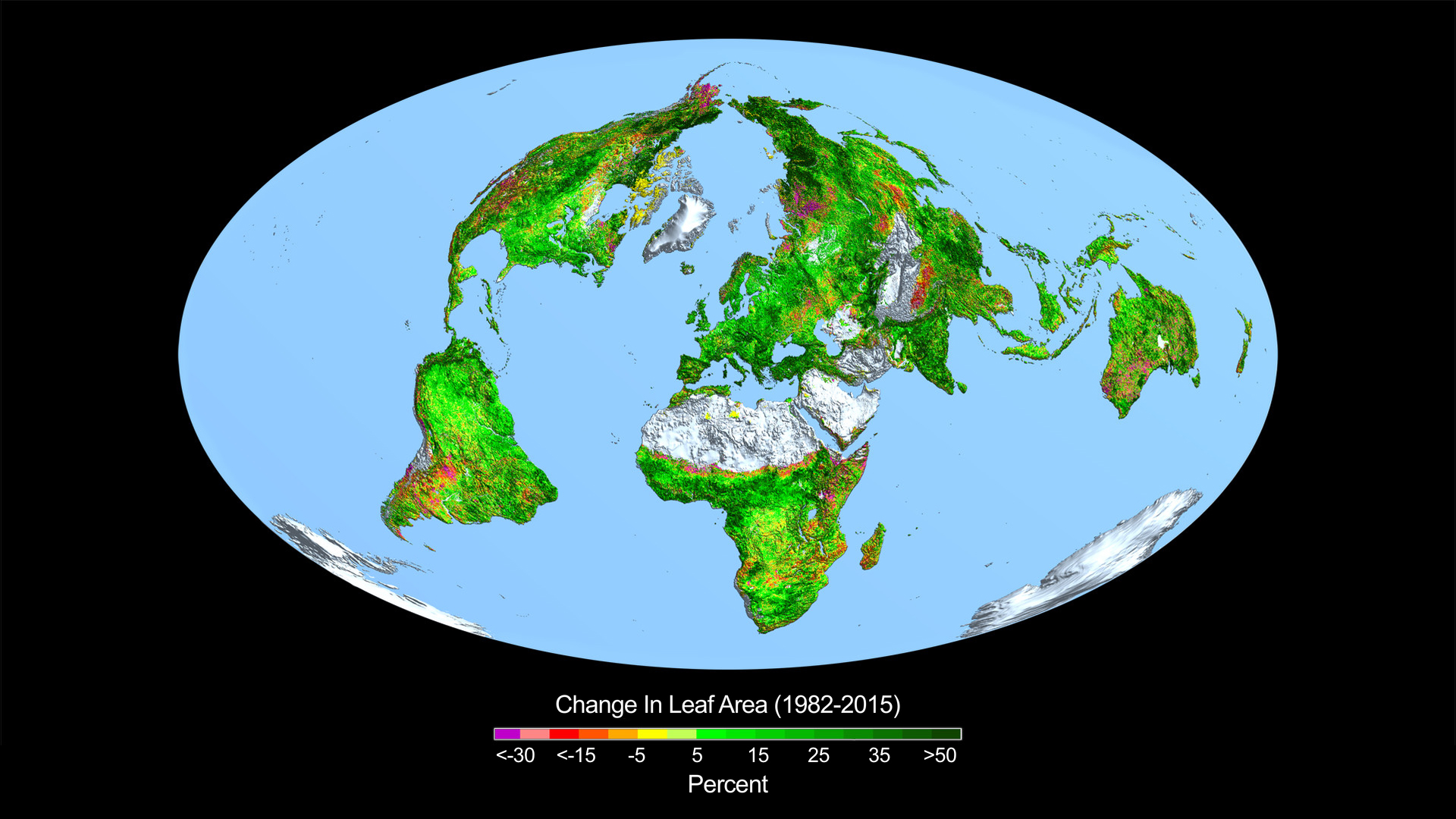
Edited on 24-03-2022 14:42 |
| 24-03-2022 19:49 | |
| Into the Night (21597) |
duncan61 wrote:Into the Night wrote:duncan61 wrote:tmiddles wrote:duncan61 wrote:...2 problems... Time to button up! We are, of course, seeing spring arrive in the northern hemisphere. Were taking our jackets off while you are putting yours on. The Parrot Killer Debunked in my sig. - tmiddles Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit nuclear powered ships do not require nuclear fuel. - Swan While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 24-03-2022 19:50 | |
| Into the Night (21597) |
Tim the plumber wrote:sealover wrote: Sorry dude. Not possible to measure either 'leaf coverage' or the global atmospheric content of CO2. The Parrot Killer Debunked in my sig. - tmiddles Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit nuclear powered ships do not require nuclear fuel. - Swan While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan Edited on 24-03-2022 19:51 |
| RE: Photorespiration if CO2 is too low. And it's hot.24-03-2022 22:13 | |
| sealover★★★★☆ (1247) |
Photorespiration if CO2 is too low. And it's hot. Photorespiration diminishes net primary productivity by about a quarter. Or at least it USED TO back in the 19-350s. Photorespiration had never even been an issue of concern until a few million years ago. Never an issue of concern unless the competition for carbon dioxide was absolutely fierce, like a rainforest, and temperatures were high. Until a few million years ago, there weren't many niches for a plant that could remain productive during CO2 drawdown at high noon, when it is so hot. Especially if the adaptation to do so made one LESS competitive under any other conditions. Photorespiration occurs when the enzyme RuBisCO accidentally attaches to a molecule of oxygen, O2. Rubisco is supposed to attach to carbon dioxide, CO2. Rubisco has much much higher affinity for CO2 than is does for O2. But when CO2 draws down to loss, a few oxygen slip through the gate because the carbon dioxide are so hard to find. When RuBisCO attaches oxygen instead of CO2, it costs the plant photosynthate. Not the FUTURE photosynthate that would have been formed if Rubisco captured CO2 like it was supposed to. Photorespiration costs the plant photosynthate that it already synthesized. Instead of taking carbon dioxide in, the plant is putting carbon dioxide out, at the cost of its own stored food supply. Imagine an indoor grower who is unaware of this. They have been gifted a somewhat large and healthy plant. They turn on the grow lights in the closet. They seal the closet door for security. The plants holds on as best it can. Hour after hour, photorespiration burns up the plants store of food, while preventing it making any new food to replace it. At some point someone notices something went wrong. Must have been the temperature, like a sauna in there. Well, yes! The temperature played an important role aggravating the damage. At any given (depleted, draw down) concentration of carbon dioxide, there is more photorespiration at higher temperature. ------------------------------------------------------------------- sealover wrote: |
| 24-03-2022 23:57 | |
| Into the Night (21597) |
sealover wrote: No such thing as 'photorespiration'. Buzzword fallacy. The Parrot Killer Debunked in my sig. - tmiddles Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit nuclear powered ships do not require nuclear fuel. - Swan While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| RE: Let me keep hanging myself with my own words. You win debate by default!25-03-2022 00:54 | |
| sealover★★★★☆ (1247) |
Let me keep hanging myself with my own words. You win debate by default. See? You don't even have to put forward an argument! I just keep hanging myself with my own words. And you can PROVE it! I'm just making this too easy aren't I? All you have to do is say, "No, it's not." All you have to do is say, "No science." All you have to do is say, "No argument." All you have to do is say, "Science is not climate" All you have to do is list a few fallacies. You are winning this thing in a blow out! Your argument wins the day without even having to show up. You've got this debating stuff down to a fine science. ------------------------------------------------------------- Into the Night wrote:sealover wrote: |
| 25-03-2022 01:49 | |
| Into the Night (21597) |
sealover wrote: Spamming. Trolling. No argument presented. The Parrot Killer Debunked in my sig. - tmiddles Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit nuclear powered ships do not require nuclear fuel. - Swan While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| RE: Vicious FIRE Feedbacks. "Firenados" The Swamp is Now in Flames.29-03-2022 23:16 | |
| sealover★★★★☆ (1247) |
Vicious FIRE Feedbacks. "Firenados". The Swamp is Now in Flames. One of the vicious feedbacks to Anthropogenic Global Weirding that I am most familiar with is in regard to the occurrence of wildfire in forests. Forest soil was my specialty forever in the Ivory Tower, until I got into coastal wetland groundwater investigation for the private sector. The mortality rates for trees in forest was already skyrocketing in the 1990s. Pests on forest trees were able to able to extend their range to higher elevations as the winters grew warmer and shorter. Trees killed by pests became a fire hazard. The presence of so many pest-killed dead trees was a major contributing factor to how destructive the wildfires were, regardless of whether or not climate change caused the fire itself. Climate change caused the pest-killed trees to contribute to the fire hazard. The fires themselves have become spectacular. "Firenados", tornado-like columns of flame towering into the sky. This wasn't my grandfather's weather any more. And can you imagine that the SWAMPS ARE AFLAME. Weird, but true. Things get too warm and dry. Even the swamps can dry up. And they can BURN. And it's hard to put peat fires out. And can you imagine RAIN FORESTS BURNING. Aren't they supposed to be pretty wet all the time? There is a HUGE amount of organic carbon stored in the biomass and soil organic matter of rain forests. It caught on fire a little bit, once or twice a century, in the good old days of 350 ppm CO2. Rainforests are burning every year now. LOTS OF CARBON DIOXIDE RELEASED. A vicious fire feedback for Anthropogenic Global Weirding. ----------------------------------------------------------------------------- sealover wrote: |
| RE: Tundra Methane: Fire REDUCES GREENHOUSE GAS IMPACT.30-03-2022 00:20 | |
| sealover★★★★☆ (1247) |
Tundra Methane: FIRE REDUCES GREENHOUSE GAS IMPACT. Under the tundra is an enormous reservoir of organic carbon in the soil organic Under the tundra is also an enormous reservoir of organic carbon in methane locked in the ice. Folks have been posting videos of setting off tundra methane with a cigarette lighter. Ironically, it HELPS TO BURN THE FOSSIL FUEL WHEN ITS METHANE. If the tundra methane floats into the atmosphere as CH4, it has about 20 as much global warming potential than it would if it had been burned and oxidized to carbon dioxide, CO2. Too bad we can't send all the tree huggers to go around the tundra with lighters. They could reduce the global warming potential of methane emissions by 95%. But there aren't enough tree huggers truly committed enough to do it. Who else could help us. METHANE OXIDIZING BACTERIA. Wherever methane comes up to meet the atmosphere, there is usually a methane oxidizing bacteria waiting for it. At the interface where oxygen is available in contact with the methane emitted, a population of methane oxidizing bacteria is usually present. Reducing the global warming potential of that methane by 95%. Thank God for methane oxidizing bacteria. Maybe we should culture them and use them to vaccinate methane leaks where fracking for natural gas has created new niches for methane oxidizers. Reducing the global warming potential of that methane by 95%. ---------------------------------------------------------------------------- sealover wrote: |
| 30-03-2022 00:46 | |
| IBdaMann (14407) |
seal over wrote:Let me keep hanging myself with my own words. You win debate by default. See? ... and you find it frustrating because you don't know what to do ... because you won't listen to those who are trying to help you by telling you what you need to do. seal over wrote:You don't even have to put forward an argument! Once again you broadcast that you are a dropout who has other people doing his thinking for him. Any educated person would know the he who makes an affirmative assertion bears the full burden of support and that nobody else is somehow required to prove the assertion false or to present any sort of counterargument. The fact that you are entirely inept at discussion reveals that your purpose for being here is not to learn. The fact that you don't know what you are talking about and that you are too stupid to learn makes it clear that you cannot possibly be here to teach anything. That leaves trolling as the only remaining reason for your presence. seal over wrote:I just keep hanging myself with my own words. Correct. seal over wrote:And you can PROVE it! No, YOU prove it. seal over wrote:I'm just making this too easy aren't I? You have no idea. Cheers.  |
| 30-03-2022 01:40 | |
| Into the Night (21597) |
sealover wrote: A normal event. sealover wrote: Making up stories about yourself still I see. sealover wrote: Argument from randU fallacy. Making up numbers and using the as data is a fallacy. sealover wrote: Winters haven't gotten shorter. Winter is still the same number of days, from Dec 21 to March 21. It is not possible to measure the temperature of the Earth or even a hemisphere of it. It isn't even possible to measure the temperature of the SDTC. sealover wrote: So? The SDTC no longer sprays to control the pests. sealover wrote: Climate cannot change, so it cannot have started any fires. Most wildfire in the SDTC is caused by arsonists. sealover wrote: Climate cannot change. Dead trees are a fire hazard. They do not have to be caused to be a fire hazard. sealover wrote: Yup. Too bad the SDTC no longer removes such fuel like they used to. sealover wrote: That is normal in any reasonably large fire. They are called 'fire pillars'. sealover wrote: Why would yesterday's weather be the same as today's weather? sealover wrote: So? Swamps burn from time to time. sealover wrote: They are fought the same as any other wildfire. sealover wrote: No. Forest fire is a normal event. sealover wrote: Soil in rain forests is extremely poor in nutrients. It's all in the vegetation. sealover wrote: Nope. Every year there are forest fires in rain forests, dead trees that burn in the SDTC, and wildfires in the SDTC grass and brushlands. Most fires in the SDTC are started by arsonists. sealover wrote: So? Why are you so afraid of carbon dioxide? It has no capability to warm the Earth. sealover wrote: There is no feedback. A wildfire is not a feedback of any kind. The Parrot Killer Debunked in my sig. - tmiddles Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit nuclear powered ships do not require nuclear fuel. - Swan While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 30-03-2022 01:44 | |
| Into the Night (21597) |
sealover wrote: Carbon is not organic. sealover wrote:Methane is not organic. sealover wrote: Things are little slow in Alaska. sealover wrote:Fossils don't burn. We don't use them for fuel. Methane is not a fossil. sealover wrote: No gas or vapor has the capability to warm the Earth. You can't create energy out of nothing. You are are still ignoring the 1st law of thermodynamics. The Parrot Killer Debunked in my sig. - tmiddles Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit nuclear powered ships do not require nuclear fuel. - Swan While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| RE: "Acid Rain" Impact on Organic Anions in Forest Floor.30-03-2022 23:11 | |
| sealover★★★★☆ (1247) |
"Acid Rain" Impact on Organic Anions in Forest Floor Picture a laboratory where a soil column is about to be sprayed with pH 3 solution of 50-50 sulfuric acid and nitric acid. Other soil columns had already been sprayed with pH 5.6 naturally acidic rain. The naturally acidic rain droplets stuck to the decomposing litter on the top of the soil columns. The litter soaked it up like a sponge. Once the litter on top of the columns was wet enough, it would begin to drain forest floor leachate into the mineral soil. NOT THE SAME WITH ACID RAIN! When columns were wetted with pH 3 50-50 sulfuric acid and nitric acid, the droplets did NOT stick to the decomposing litter on top of the soil columns. There was a hydrophobic effect as droplets beaded off the top of the litter and flowed directly down to the mineral soil. It took a long time for the litter to wet up enough to act as a sponge. Much of the acid rain entered the mineral soil without interacting with the litter. This made a big difference to what happened in the soil as the "raw" acid rain percolated down the soil column. With naturally acidic rainfall, the forest floor leachate was full of organic anions. These organic anions could form strong complexes with calcium and magnesium. These organometallic complexes of calcium and magnesium could adsorb to organic matter in the soil to be retained against leaching loss. With "acid rain", far fewer organic anions are present in forest floor leachate. Protonation of organic anions by "acid rain" renders many of them insoluble. Protonation of organic anions by "acid rain" greatly diminishes their capacity to form organometallic complexes with calcium and magnesium. So, they don't. Instead, calcium and magnesium are complexed by SULFATE from the sulfuric acid in "acid rain". To a lesser extent, NITRATE drags off some calcium and magnesium too. So, calcium sulfate, magnesium sulfate, calcium nitrate, and magnesium nitrate all wash away from the forest soil where the trees need them. The calcium and magnesium should have been picked up and recaptured by organic anions. They would have stayed in the soil to get back into the trees. But "acid rain" screwed everything up. ---------------------------------------------------------------------------- sealover wrote:Into the Night wrote:sealover wrote: |
| RE: Oligotrophic Rainforests on Acid White Sands. Biomass versus Soil Carbon Content.30-03-2022 23:45 | |
| sealover★★★★☆ (1247) |
Oligotrophic Rainforests on Acid White Sands. Biomass versus Soil Carbon Content. "Soil in rain forests is extremely poor in nutrients. It's all in the vegetation." Sometimes true, sometimes not. Rainforests on soils developed from recent volcanic ash or volcanic mudflows can be extremely RICH in nutrients. Those guys are EASY to reforest. Those guys take many many harvests of slash and burn before you start to diminish their productivity. Rainforests in delta regions where flooding has just lay down a new layer of nutrient rich sediment - the topsoil washed off from some land upriver. Those rainforest soils are extremely rich in nutrients. Rainforests in downslope positions, where landslides lay down nutrient rich topsoil from above. Those rainforest soils can have a lot of nutrients. At the opposite extreme are the rain forests on acid white sand. In those forests, the mineral soil is EXTREMELY POOR in nutrients. Acid white sand soils are so devoid of nutrients that roots don't even grow in the mineral soil. It's all in the vegetation, or in the deep litter layer that the vegetation has created above the acid white sand mineral soil. So as far as NUTRIENTS go, YES they are all in the live or dead biomass. Same with the carbon. There is no organic carbon stored in the acid white sand mineral soil. All the organic carbon is in the live or dead biomass ABOVE the mineral soil. Now, where is MOST of the organic carbon? In the LIVE BIOMASS? NO! Most of the organic carbon in that rainforest on the acid white sand is contained in the dead organic matter above the mineral soil surface. Indeed, as is the case with MOST FORESTS, there is A LOT MORE ORGANIC CARBON IN DEAD BIOMASS THAN LIVE BIOMASS. ------------------------------------------------------------------- Into the Night wrote: Soil in rain forests is extremely poor in nutrients. It's all in the vegetation. |
| RE: Sustainable Slash and Burn. Black Ash versus White Ash.31-03-2022 00:34 | |
| sealover★★★★☆ (1247) |
Sustainable Slash and Burn. Black Ash versus White Ash. Paul Zinke was an excellent forest soil science professor at UC Berkeley. Much of his own research was in the rainforests of Southeast Asia, where indigenous tribe people practiced slash and burn on the hillslopes of the rain forest. Slash and burn on an inclined hill slope exposes the barren soil to erosion. But they way these folks did it was SUSTAINABLE. They were very careful preparing the fuel before setting the fires. Branches of felled trees were cut and laid out along the contours of the slope, to minimize erosion. Fuel was carefully distributed and the fuel moisture carefully monitored. When conditions of fuel moisture, temperature, wind, time of day were all just right, the wise old man knew it was time to strike the matches. The fire was low intensity. It left BLACK ASH. NOT white ash. The difference between a fire leaving black ash versus white ash is HUGE as far as nutrient losses or risk of erosion goes. In northern California, one of the most important jobs of the tribal wise man was to know when to set the fires. Done while the fuel was still moist in the spring. A low intensity fire, not like the wildfires from dry lightening at the end of the summer - white ash conflagrations. A deliberately set, black ash low intensity fire, to open up for a fresh new crop of deer feed. A deliberate low intensity fire to make the acorns easier to find, and to give the acorns a better shot at growing up to make more acorns. Anthropogenic shift in dominance of acorn bearing oaks in California foothills. A deliberate low intensity fire to make the pine nuts easier to find, and to give the close coned pines the heat they need to open up and let out their seed to make more pine nut bearing trees. Anthropogenic shift in dominance of pine nut bearing closed cone pines in California foothills. So, the tribesmen in Southeast Asia, according to the royal tax records, their dry rice harvests had been consistently high on the same land for centuries. Sustainable slash and burn farming in rainforests. It's just a little labor intensive, but it can be done. The most important trick is to ensure a LOW INTENSITY FIRE. Black ash is better than white ash. A LOT better. ------------------------------------------------------------------ sealover wrote: |
| 31-03-2022 18:09 | |
| GretaGroupie (350) |
IBdaMann wrote: Oh, my, IBM, that looks interesting. Why'd he melt down? [ADDED ABOUT 40 MINUTES LATER] - Wow, I clicked on that lunatic link you posted, IBM, and this trafn wrote a lot of books. He even wrote one to Greta! I ordered both of them. I'm sure I'll have lots of questions. I'm so glad I joined here!!!! Edited on 31-03-2022 18:50 |
| 31-03-2022 20:20 | |
| IBdaMann (14407) |
GretaGroupie wrote:Oh, my, IBM, that looks interesting. He's compulsive paranoid obsessive. Otherwise, he's a good chap. He just cannot stand the thought of a differing viewpoint residing on the same board. He absolutely needed to have all differing views removed from everywhere on the board. To accomplish this, he began massive spamming attacks on all threads in his attempt to drown out and ultimately bury all expression of differing views. I was a major purveyor of differing views. trafn really did not like me. Again, he otherwise seems like a good guy. |
| RE: Why the "Hockey Stick" graph should NEVER have been included.31-03-2022 21:16 | |
| sealover★★★★☆ (1247) |
Why the "Hockey Stick" graph should NEVER have been included. The infamous "Hockey Stick" graph. A straight flat line suddenly angles up into straight inclined line. Nature doesn't work that way. It is unfortunate that this grossly oversimplified presentation was ever included. Indeed, if the "Hockey Stick" graph had used shorter time intervals for averaging, something would have emerged that better predicted the temperature increases of the last 30 years. There was NEVER a straight flat line for average temperature over centuries. And the temperature rise has NEVER been a straight line incline up. If the temperature data, or actually the ESTIMATED temperature data had been averaged over shorter time interval, the preindustrial temperatures would have been sort of a sine wave going up and down. What we got since the "Hockey Stick" was published are the temperature numbers for three decades of net increase. They don't look like the end of a hockey stick. They look more like a stair case. During periods of consecutive years when the natural trend was a slight temperature decrease, the anthropogenic increase was largely canceled. Those are the flat step parts of the staircase. During periods of consecutive years when the natural trend was a slight increase, the anthropogenic increase was AMPLIFIED! Those are the very steep increases between steps in the staircase. People who were expecting to see a hockey stick instead saw a sine wave angle up to become a staircase. It appeared to DEFY predictions of global warming when, in fact, it CONFIRMED them. Some of those idiots insisted that we had GLOBAL COOLING during the consecutive years when natural cooling canceled anthropogenic forcing. So, the "Hockey Stick" didn't do anyone any favors as far as providing an accurate prediction of future, well now past and present, temperature trends. ------------------------------------------------------------------------------------- IBdaMann wrote:GretaGroupie wrote:Oh, my, IBM, that looks interesting. |
| RE: "Acid Rain". Aluminum Toxicity. Organic Anions. Calcium.31-03-2022 23:18 | |
| sealover★★★★☆ (1247) |
"Acid Rain". Aluminum Toxicity. Organic Anions. Calcium. Many forest soils form from silica-rich parent material such as granite. They support evergreen, sclerophyllous woody perennials. Needle leaf forests and chaparral are what can survive in the very low calcium soil. Pines, among others, evolved to need only the tiniest amounts of calcium. In contrast, forest soils formed from limestone support a very different kind of plant community, different growth habit and morphology. Neutral pH soils rich in calcium. "Acid Rain" was harmless to forests on calcareous soils. "Acid Rain" was devastating to forests on acidic, siliceous soils. Calcium deficiency and aluminum toxicity were identified as the culprits. Aluminum toxicity can be a major limitation to crop productivity on soils with pH less than 5, following deforestation. Lime is often applied to ensure pH is no less than 5, or more ideally, at least 5.5 to avoid aluminum toxicity. However, before deforestation, the forest was thriving on that same soil with pH less than 4. To plants adapted to acidic soils, aluminum is only toxic when it is in labile ion form in solution. Aluminum that is complexed by organic ligands is NOT toxic. Before the forest was cleared, the forest floor supplied organic anions that leached into the mineral soil. These anions complexed calcium and magnesium, to prevent their loss from the ecosystem. Before clearing the forest, organic anions from the forest floor formed organometallic complexes with labile aluminum in solution, rendering it non toxic. Before "acid rain", organic anions from the forest floor formed organometallic complexes with labile aluminum in solution, rendering it non toxic. "Acid rain" causes organic anions to become protonated into organic acids. This makes many of them insoluble. This makes those that are still soluble far less capable of forming organometallic complexes with aluminum to detoxify it. There is also a synergistic effect between calcium deficiency and aluminum toxicity. Curing one also cures the other. --------------------------------------------------------------------------------- sealover wrote: |
| 01-04-2022 00:21 | |
| Into the Night (21597) |
sealover wrote: What do you mean by 'forcing'? How do you know there was 'natural cooling'? It's not possible to measure the temperature of the Earth. The Parrot Killer Debunked in my sig. - tmiddles Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit nuclear powered ships do not require nuclear fuel. - Swan While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 01-04-2022 00:25 | |
| Into the Night (21597) |
sealover wrote: Solubility isn't affected by pH. Rain is normally acid. The Parrot Killer Debunked in my sig. - tmiddles Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit nuclear powered ships do not require nuclear fuel. - Swan While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan Edited on 01-04-2022 00:26 |
| RE: "Solubility isn't affected by pH. Rain is normally acid."01-04-2022 01:38 | |
| sealover★★★★☆ (1247) |
"Solubility isn't affected by pH. Rain is normally acid." Yes, hidden in the fine print of the post was reference to "naturally" acidic rain with a pH about 5.6. This "normally acid" pH 5.6 rain is acidic only from CARBONIC ACID. Also hidden in the fine print of the post, the "acid rain" was pH 3, 50-50 sulfuric and nitric acid. Not the "normally acid" kind. The ANTHROPOGENIC kind. In 1990, sealover's master's thesis was entered into the UC Berkeley Library. The title: Phenols in forest litter and leachate: Interaction with Acidity. It was ALL ABOUT how solubility IS affected by pH. First, the hydrophobic effect of increased surface tension in the rain drops. The high ionic strength of the pH 3 "acid rain" means that there is high surface tension in the rain droplets. This prevents some of it from even interacting with with the litter before percolating down into mineral soil. "Normally acid" rain does NOT do this. The solubility of phenol carboxylic acids is HIGHLY pH dependent. As deprotonated anions, they can form dipoles with surrounding water molecules lined up in polar fashion. This makes them soluble despite their hydrocarbon component, which tends to be hydrophobic. That master's thesis was a very rare case of one that outsiders went to see at the UC Berkeley library. Between 1990 and 1994, there was no place else to find the information it contained. In fact, pH is the MASTER VARIABILITY regulating the solubility of organic matter in water. ------------------------------------------------------------------------ Into the Night wrote:sealover wrote: |
| 01-04-2022 03:10 | |
| duncan61★★★★★ (2021) |
sealover wrote: I worked in the Tully rainforest in the military and it is not possible to light fires.Even the Hexamine blocks we were issued would not light.Where are rainforests burning?I have been measuring CO2 with a meter for nearly 12 months and it has gone down about 30ppm.Please explain? duncan61 |
| 01-04-2022 05:03 | |
| IBdaMann (14407) |
seal over wrote:Why the "Hockey Stick" graph should NEVER have been included. It is unfortunate that this grossly oversimplified presentation was ever included. Included? You used the word "included" in reference to a data fabrication. Are you referring to the inclusion of said data fabrication into official Church dogma? seal over wrote:Indeed, if the "Hockey Stick" graph had used shorter time intervals for averaging, something would have emerged that better predicted the temperature increases of the last 30 years. Math is not your strong suit. Why do you believe that shorter time intervals would yield anything useful? Let's talk math, shall we? Yes, we shall. For any time interval, to determine the increase or decrease in temperature over that time interval, I only need two data points, i.e. the beginning and the end of the time interval, and the subtraction operation. Two data points and subtraction give one the exact temperature change. Also, you used the word "predicted" ... as though your religion has any ability to predict nature. I think you meant to use the word "divine" the future and impart omniscience upon believers. Yes, I believe that is what you meant to write. Why do you believe there were any "temperature increases" over the last thirty years that aren't explained by normal changes of the weather, by changing of the seasons or by night changing into day and vice-versa? seal over wrote:There was NEVER a straight flat line for average temperature over centuries. How do you know? You don't know what the average planetary temperature was, ever. You don't know if it ever changed. Yes, you believe that it changed, and you believe religiously ... but you don't know. Your faith does not impart any of the omniscience you believe it does. seal over wrote:And the temperature rise has NEVER been a straight line incline up. Another omniscience fallacy. seal over wrote:If the temperature data, or actually the ESTIMATED temperature data There is no The Data. There has never been any The Data. You have been deceived. From The MANUAL The Data: proper noun According to Global Warming mythology, The Data is the rumored proof of Global Warming, the mere mention of which has the magical superpower to end all debate on questions of Global Warming faith. Note: Often Climate Scientists fabricate data and claim that it comes from The Data. As long as the fabricated/cooked/tweaked/modified/fudged/altered/fiddled data support the truth of Climate Science then it is the Climate Scientists' duty to present that nondata. This duty is analogous to Taqiya in Islam. seal over wrote: the preindustrial temperatures would have been sort of a sine wave going up and down. Why is the Karl Marx timeline, centered around the Industrial Revolution, of any relevance? It's almost as if Global Warming is specifically out to attack capitalism. Weird. seal over wrote:During periods of consecutive years when the natural trend was a slight temperature decrease, the anthropogenic increase was largely canceled. You raise a great question. I'm going to shotgun this question out to GasGuzzler because of his recognized Climate acumen (for which he has won many awards and bonus points): When I take two temperature measurements and perform a subtraction operation in order to calculate the exact temperature change, how can I accurately distinguish the anthropojanitor component of that temperature change from the unanthropojanitor component? seal over wrote:During periods of consecutive years when the unanthropojanitor trend was a slight increase, the anthropojanitor increase was AMPLIFIED! ... and all this is in The Data? Please send me a copy of this The Data so I can drop it into Excel and run my analysis? Just send me the The Data that you are using. seal over wrote:Those are the very steep increases between steps in the staircase. I can't wait to see the charts in Excel! seal over wrote:People who were expecting to see a hockey stick instead saw a sine wave angle up to become a staircase. I won't tell you what I expect. seal over wrote:It appeared to DEFY predictions of global warming when, in fact, it CONFIRMED them. ... because that's what science does, right? seal over wrote:Some of those idiots insisted that we had GLOBAL COOLING during the consecutive years when natural cooling canceled anthropogenic forcing. Forcing! Yessss! Those anthropojanitors are not to be messed with! . |
| 01-04-2022 05:47 | |
| duncan61★★★★★ (2021) |
sealover wrote: -------------------------------------------------------------- "Rainfall is naturally acid". Yes, it is. In fact, you can calculate the pH of natural rainfall just by knowing the atmospheric carbon dioxide concentration. Don't be afraid! It can be intimidating at first, but science math can also be fun. Back when atmospheric CO2 was about 350 ppm, rainfall pH was about 5.6. Scientists could even predict how much the rainfall pH would drop when the concentration of CO2 in the atmosphere reached 360 ppm. That is because carbonic acid in the raindrops is in chemical equilibrium with carbon dioxide gas in the atmosphere. But "acid rain" isn't about carbonic acid. Most of the acidity in "acid rain" came from sulfuric acid. Also known as hydrogen sulfate. In some regions, most of the acidity in "acid rain" came from nitric acid. Also known as hydrogen nitrate. One of the adverse environmental impacts of "acid rain" was aluminum toxicity. The trees weren't actually "eating" the aluminum. Aluminum exists in bauxite and is removed by digestion in Caustic soda then the alumina powder is extracted and then smelted to aluminum. I have no reason to believe there are lumps of aluminium in the ground naturally occurring. alumina powder is the grindy stuff in toothpaste and is completly harmless to humans duncan61 |
| 01-04-2022 07:18 | |
| GasGuzzler★★★★★ (2933) |
seal over wrote:During periods of consecutive years when the natural trend was a slight temperature decrease, the anthropogenic increase was largely canceled. IBdaMann wrote: Thank you for giving me the stage on this one. Estimated data analytics really is my field of scientific expertise. We KNOW that the average global temperature has risen 0.1 degree C every decade since grandma passed away in '73. For all practical purposes it's been close enough to five decades now, we'll just call it 70 years. Fair enough? Good. Let's continue. A tenth of a degree every decade for 8 decades gives you 8 tenths of one degree. Simple, right? Now, for simplified guesstimation, we have nearly 1 degree Celsius rise in global temp since grandma died 50 years ago. Precision extrapolation gives us a final global temperature rise of 2 degrees per century, or .02 per year. How are we doing? Everyone following along OK? (Sorry, sometimes my target audience tends to wander in and out of consciousness.) Now comes the tricky part. We must calculate the variable forcings with our baseline rise of .03C per year. To do this we gather our list of forcings and assign each one a specific coefficient value, assuming of course each forcing is an accurately estimated constant. Here we go. element / coefficient value CO2 .025 Methane .0137 Ferns .028 Carburetor acid .029 Tanning Beds .0035 Chlorofluorocarbons .023 AA alkaline Batteries .009 IBdaMann insult- per occurrence. 2.9203 So the final step is really easy. Add the average coefficient values together, divide by the square root of our baseline yearly average temperature rise (.04C), adjust upward another .005 degrees to account for the dirty orbit of the satellite collecting temperature data, and voila! Peanut Butter Chicken! ...filed in my library. Cheers! |
| 01-04-2022 16:45 | |
| gfm7175 (3314) |
GasGuzzler wrote:seal over wrote:During periods of consecutive years when the natural trend was a slight temperature decrease, the anthropogenic increase was largely canceled.IBdaMann wrote: WOW. I am completely awestruck by your wisdom on this topic. Truly mind blowing stuff here! It seems to me that we could significantly slow down the rate of average global temperature increase if we would simply implement both an IBdaMann insult tax and an IBdaMann insult sequestration policy. Together, those transitory government policies would reduce the average global temperature anomaly, significantly reduce unprecedented extreme weather events, and save the polar bears from extinction. We need to ACT NOW!!! Faster than Xadoman buying into the latest cryptoscam!! |
| 01-04-2022 17:08 | |
| GasGuzzler★★★★★ (2933) |
gfm7175 wrote:GasGuzzler wrote:seal over wrote:During periods of consecutive years when the natural trend was a slight temperature decrease, the anthropogenic increase was largely canceled.IBdaMann wrote: Yes! We build an IBdaMann sequestration pipeline and pump that bullshit 10,000 ft down. This way we bury his shit forever. He's such an athole! Radiation will not penetrate a perfect insulator, thus as I said space is not a perfect insulator.- Swan |
| 01-04-2022 18:17 | |
| GretaGroupie (350) |
IBdaMann wrote:GretaGroupie wrote:Oh, my, IBM, that looks interesting. Oh, that's a shame, but I got big news. I have a friend who owns a bookstore and he had a used copy of the bursting book ($3, what a bargain!), so I read it last night. Turns out it's quite short and only took about 2 hours. It wasn't too hard to follow, and the math was pretty basic, but I got confused by the whole "apple core" thing that had something to do with the northern lights. From what I did understand of it, I'm not saying what he wrote is impossible, but in the world of jelly beans, it's a blue one. My friend didn't have a copy of his Greta book, so I ordered it on Amazon. I'll keep you posted. |
| 01-04-2022 20:13 | |
| Spongy Iris (1643) |
GretaGroupie wrote: Nice to meet you GretaGroupie! Speaking of the northern lights, I came across this article on Space.com a couple days ago: https://www.space.com/sun-erupts-sunspot-17-solar-flares Supposedly the Sun should have made some pretty big coronal mass ejections these past 2 days. There should have been some geomagnetic storms that came with it, but they say the northern and southern lights are hard to predict, so I don't know. I'm not close enough to the northern and southern lights to be able to tell. %20(1).png)
Edited on 01-04-2022 20:14 |
| 01-04-2022 20:35 | |
| Into the Night (21597) |
...fixing severely damaged quoting that you STILL haven't figured out how to use...sealover wrote:Into the Night wrote:sealover wrote: It also naturally contains nitric acid and sulfuric acid. Nitric acid is formed from naturally occurring nitrogen dioxide gas and rainwater. Sulfuric acid is formed from naturally occurring sulfur dioxide gas and rainwater. Both gases occur naturally in the atmosphere. sealover wrote: There is no 'kind'. Sulfuric and nitric acid are normally found in rain. sealover wrote: 1) I don't believe you. 2) If you ever do write such a thesis, I would flunk it, since such a paper ignores naturally occurring sources of acid in rainwater, ignores why water drainage to rivers makes the water alkaline, and ignores that the ocean is alkaline. I assume your conclusion would also claim 'global warming' somehow and would therefore ignore the 1st law of thermodynamics. You really gotta stop making up shit about yourself dude. You are a nothing. You deny science. You do not understand chemistry, including acid-base chemistry. You show it with every post. You cut and paste with no understanding of the material you are cutting and pasting. You are a nothing. sealover wrote: Solubility is not affected by pH. Someday you will learn about the rules of solubility. Nah...who am I kidding? sealover wrote: So....rain doesn't soak into soil, eh? The grass, shrubs, and trees are going to get very disappointed... sealover wrote: Not a hydrocarbon. Did you put this in your 'paper' too? Flunk. sealover wrote: You are a nothing. Making up shit about how important you are isn't going to fly here. sealover wrote: No. You still don't understand solubility. pH is not a factor. The Parrot Killer Debunked in my sig. - tmiddles Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit nuclear powered ships do not require nuclear fuel. - Swan While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan |
| 01-04-2022 20:35 | |
| IBdaMann (14407) |
GretaGroupie wrote:Oh, that's a shame, but I got big news. Oooops, I suppose that it is too late to mention that you can get it on Climate-Debate for free. Well, it's good that you supported your friend. For future reference, trafn came to this site merely to push his view (much like seal over is doing with his own view of the world) and posted a free copy of his booka free copy of his book in his very first post. GretaGroupie wrote:My friend didn't have a copy of his Greta book, so I ordered it on Amazon. I'll keep you posted. Please do. trafn did not post a free copy of Greta's book, unfortunately. |
| 01-04-2022 20:47 | |
| Spongy Iris (1643) |
IBdaMann wrote:GretaGroupie wrote:Oh, that's a shame, but I got big news. Thank you. I was just searching for a free copy and you saved me some time. %20(1).png) |
| 01-04-2022 20:49 | |
| IBdaMann (14407) |
GretaGroupie wrote:It wasn't too hard to follow, and the math was pretty basic, but I got confused by the whole "apple core" thing that had something to do with the northern lights. trafn was using the "apple core" analogy to describe the shape of the earth's magnetic field.  Notice the specific curvature/shape at the poles. This is where the earth's magnetic field "funnels in" charged particles from the sun that create the northern and southern lights (depending on the time of year). This is entirely correct. Unfortunately, trafn uses this particular phenomenon to erroneously jump to bogus conclusions about "energy being transferred to the atmosphere." Don't be afraid to come to me with the hard stuff. . |
| 01-04-2022 20:52 | |
| Into the Night (21597) |
duncan61 wrote:sealover wrote: Farmers and ranchers in Brazil clear land of rainforest and burn the wood. Brazil has passed laws limiting this activity to protect the rainforest. It is poor land use, since the resulting open land is poor in nutrient and easily erodes. Yeah. It's tough to light wet hexamine. In Washington, on the west side of Mt Olympus, and just in from the coast about 5 miles, is a temperate rainforest. I've never heard of a wildfire there. Wildfire does occur elsewhere in Olympic park, but not in the rainforest area. The Parrot Killer Debunked in my sig. - tmiddles Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit nuclear powered ships do not require nuclear fuel. - Swan While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan Edited on 01-04-2022 20:59 |
| RE: "Acid Rain" and "Nitrogen Saturation" of Ecosystems.01-04-2022 21:46 | |
| sealover★★★★☆ (1247) |
"Acid Rain" and "Nitrogen Saturation" of Ecosystems. "Acid Rain" did a lot of damage to a lot of ecosystems. Most of the acid in "acid rain" was sulfuric acid (hydrogen SULFATE). Most of the damage was done by the strong mineral acid and its associated oxyanion, sulfate. Sulfate formed strong complexes with calcium and magnesium, removing these essential nutrients from the soil via surface runoff or leaching into groundwater. The sulfuric acid in acid rain was mostly from anthropogenic coal combustion. Lesser amounts of sulfuric acid in acid rain were from combustion of sulfur containing DIESEL fuel. Still lesser amounts of sulfuric acid in acid rain were from natural sources, such as volcanic activity or wildfires burning sulfur containing organic material. The other acid in "acid rain" was NITRIC ACID (hydrogen NITRATE). Nitric acid was becoming a larger and larger ingredient in "acid rain" as humans used more and more vehicles for transportation on the land or in the sea and sky. The heat of combustion provided activation energy to oxidize some nitrogen gas into NOx, including nitric acid. In California in the 1980s, about 2/3 of the acid in "acid rain" was NITRIC acid, and the other 1/3 was sulfuric acid. Sulfuric acid was the bad boy who dragged off the calcium and magnesium. Nitric acid didn't drag off so much calcium and magnesium, but it provided FERTILIZER. Most natural ecosystems were nitrogen limited. Most natural ecosystems would respond to added nitrogen. "Nitrogen saturation" was the buzzword they made up for this fake science. Heathlands were being taken over by faster growing plant species, no longer limited by nitrogen availability. Watersheds were "leaking" nitrate into groundwater and surface water. Plant communities were shifting in response to shifting nitrogen availability. Humans weren't doing much of anything to reduce anthropogenic nitric acid in "acid rain". But something funny happened about a dozen years ago. "sealover" was asked to review a new paper (peer review before publication kind of thing). This paper was the first of many that would find Mother Nature had adapted to "nitrogen saturation". The nitric acid (hydrogen nitrate) additions to the watershed had not ceased. Just as much nitrate was raining down into the watershed as before. But the concentration of nitrate in ground water and surface water was steadily declining toward negligible amounts. Well, before anthropogenic "acid rain", there hadn't been much of a niche available for nitrate reducing bacteria in the soil and groundwater of these watersheds. Historically, there had never been enough nitrate to support them. It took a few decades for them to get established, but now there was a population of nitrate reducing bacteria along the subsurface flow paths. They were catching the nitrate in the water and using it to oxidize organic carbon via denitrification or via dissimilatory nitrate reduction to ammonium. They were neutralizing the acidity of the nitric acid, as nitrate reduction generated alkalinity. The bacteria were consuming the excess nitrate from the ground water before it could find its way into any surface waters to cause eutrophication, hypoxia, and fish kills. Some of that nitrate was being lost as dinitrogen gas to denitrification. Most of that nitrate was being retained in the ecosystem a dissimilatory reduction of nitrate to ammonium generated alkalinity while transforming the nitrate into ammonium, which can be held in soil by cation exchange. What could we learn from this? There are other places where anthropogenic impacts cause a material to be present where it was not before. Methane coming up out of the tundra. Ammonium in the gold mine tailings being oxidized to nitrate. Nitrate from "acid rain" in subsurface flows and surface waters. Nature already had a microorganism adapted to solve the problem. But there had never been such a problem in these specific locations before. A microorganism was going to have to move in from somewhere else to oxidize the methane or consume the nitrate. That can take decades or longer. Humans can give the microbes a helping hand to put them where they needed. Humans can selectively breed or even genetically engineer the microorganisms to do an even better job cleaning up our mess for us. ---------------------------------------------------------------------------------- Spongy Iris wrote: Oooops, I suppose that it is too late to mention that you can get it on Climate-Debate for free. Well, it's good that you supported your friend. For future reference, [url=https://www.climate-debate.com/trafn-p647.php][color=blue] |
| RE: And we can select WHICH nitrate reducing bacteria to introduce. No N2 loss to denitrification.01-04-2022 22:23 | |
| sealover★★★★☆ (1247) |
And we can select WHICH nitrate reducing bacteria to introduce. No N2 loss to denitrification. So, we have the phenomenon of forested watersheds that displayed "nitrogen saturation" a few decades ago, but now they are no longer "leaking" out nitrate. Even though input of nitrate as nitric acid in "acid rain" has not ceased. Nitrate reducing bacteria eventually established active populations along the subsurface flow paths of these watersheds. Humans could have accelerated the natural mitigation. Humans could have introduced nitrate reducing bacteria, along with the rainfall entering subsurface flow paths. Humans could have tailored the selection of nitrate reducing bacteria to be a best fit for local conditions, physical and chemical. Humans could have ensured that ONLY bacteria that perform dissimilatory reduction of nitrate to ammonium are introduced. NO DENITRIFIERS! Denitrifying bacteria transform nitrate into dinitrogen gas, mostly. That nitrogen gas goes to the atmosphere where it is useless as a nutrient for ecosystems. Dissimilatory reduction of nitrate to ammonium by bacteria keeps the nitrogen in the ground as ammonium. Furthermore, there are FACTORS THAT REGULATE HOW MUCH NITROUS OXIDE is emitted as a by product during either denitrification OR dissimilatory reduction of nitrate to ammonium. We can selectively breed the bacteria. Maybe even genetically engineer them. Ensure that they put out as little nitrous oxide as possible. And ensure that the physical and chemical conditions under which they operate allow them to minimize the emission of nitrous oxide during nitrate reduction. ---------------------------------------------------------------------------- [quote]Into the Night wrote: [quote]duncan61 wrote: [quote]sealover wrote: Vicious FIRE Feedbacks. "Firenados". The Swamp is Now in Flames. One of the vicious feedbacks to Anthropogenic Global Weirding that I am most familiar with is in regard to the occurrence of wildfire in forests. Forest soil was my specialty forever in the Ivory Tower, until I got into coastal wetland groundwater investigation for the private sector. The mortality rates for trees in forest was already skyrocketing in the 1990s. Pests on forest trees were able to able to extend their range to higher elevations as the winters grew warmer and shorter. Trees killed by pests became a fire hazard. The presence of so many pest-killed dead trees was a major contributing factor to how destructive the wildfires were, regardless of whether or not climate change caused the fire itself. Climate change caused the pest-killed trees to contribute to the fire hazard. The fires themselves have become spectacular. "Firenados", tornado-like columns of flame towering into the sky. This wasn't my grandfather's weather any more. And can you imagine that the SWAMPS ARE AFLAME. Weird, but true. Things get too warm and dry. Even the swamps can dry up. And they can BURN. And it's hard to put peat fires out. And can you imagine RAIN FORESTS BURNING. Aren't they supposed to be pretty wet all the time? There is a HUGE amount of organic carbon stored in the biomass and soil organic matter of rain forests. It caught on fire a little bit, once or twice a century, in the good old days of 350 ppm CO2. Rainforests are burning every year now. LOTS OF CARBON DIOXIDE RELEASED. A vicious fire feedback for Anthropogenic Global Weirding. |
| RE: Polyphenol Reduction of Methane Emission from Bovine Burps.01-04-2022 22:46 | |
| sealover★★★★☆ (1247) |
Polyphenol Reduction of Methane Emission from Bovine Burps. "sealover" loves his buzzwords, but POLYPHENOL just might be his favorite. When cattle or other ruminants digest cellulose-rich feed, bacteria inside protozoa inside bovine guts produce a cellulase enzyme, enabling the animal host to benefit from this otherwise indigestible carbohydrate. But the low oxygen conditions of the bovine gut favor methanogenesis. Cattle burp out a WHOLE LOT OF METHANE. But cattle burp out a WHOLE LOT LESS METHANE when their feed contains the right amount of the right kind of polyphenol. Not just theoretical. Already being applied commercially. We could discuss this kind of practical, inexpensive, and quick solution. Or we could argue about whether or not we are allowed to discuss methane in any context because unambiguous definition of methane as a greenhouse gas involved in climate change, blah, blah, blah, blah, blah... ANWER THE QUESTION! What question? DEFINE YOUR TERMS! Yeah, that WAS the only real QUESTION, wasn't it? ---------------------------------------------------------------------------- --------------------------------------------------------------------------- IBdaMann wrote:seal over wrote:Let me keep hanging myself with my own words. You win debate by default. See? |
| RE: Google "UC Berkeley Library Phenols in forest litter and leachate: Interaction with Acidity"01-04-2022 23:20 | |
| sealover★★★★☆ (1247) |
Google "UC Berkeley Library Phenols in forest litter and leachate: Interaction with Acidity" SOMEBODY published the assertion that solubility IS affected by pH more than 30 years ago. It is fine if you still believe I am a "liar". YOU ARE OBVIOUSLY NOT THE TARGET AUDIENCE. Your belief or disbelief is irrelevant to the reality being discussed. The fact that pH DOES have ENORMOUS influence on the solubility of organic acids is very important to understanding their behavior. We KNOW that liming acid sulfate soils causes HUGE increase in their export of dissolved organic matter to surface waters. Same for liming siliceous acidic soil forested watersheds. Same for liming strongly acidic dredged sediment. Indeed, the very definition of "humic acid", OPERATIONALLY DEFINED, of course. Unambiguous definition of "humic acid" - the fraction of organic matter that is INSOLUBLE IN STRONG ACID. Everything but humic acid is soluble in the strongest acid. All the carbohydrates and everything else hydrolyzed or dissolved or burned... But the humic acids are the gigantic polymers of organic anions that become fully protonated and insoluble in strong acid. pH dependence of organic anion solubility? HELL YES! ------------------------------------------------------------- Into the Night wrote: Solubility isn't affected by pH. Rain is normally acid.[/quote] sealover wrote: 1) I don't believe you. 2) If you ever do write such a thesis, I would flunk it, since such a paper ignores naturally occurring sources of acid in rainwater, ignores why water drainage to rivers makes the water alkaline, and ignores that the ocean is alkaline. I assume your conclusion would also claim 'global warming' somehow and would therefore ignore the 1st law of thermodynamics. You really gotta stop making up shit about yourself dude. You are a nothing. You deny science. You do not understand chemistry, including acid-base chemistry. You show it with every post. You cut and paste with no understanding of the material you are cutting and pasting. You are a nothing. sealover wrote: Solubility is not affected by pH. Someday you will learn about the rules of solubility. Nah...who am I kidding? sealover wrote: So....rain doesn't soak into soil, eh? The grass, shrubs, and trees are going to get very disappointed... sealover wrote: Not a hydrocarbon. Did you put this in your 'paper' too? Flunk. sealover wrote: You are a nothing. Making up shit about how important you are isn't going to fly here. sealover wrote: No. You still don't understand solubility. pH is not a factor.[/quote] |
| 02-04-2022 00:27 | |
| Into the Night (21597) |
...deleted severely damaged quoting...you still can't figure out how to quote on forums...sealover wrote: Not possible to measure, dude. You can't measure rain globally or even regionally. You are making shit up again. Argument from randU fallacy. sealover wrote: There is no such chemical as 'sulfate'. Calcium is not a nutrient and doesn't even occur in nature. Magnesium is not a nutrient and doesn't even occur in nature. sealover wrote: Making up numbers again. Argument from randU fallacy. Coal is carbon, not sulfur. sealover wrote: Argument from randU fallacy. You are still making up numbers. sealover wrote: Sulfur isn't organic. sealover wrote: I already said this. sealover wrote: Neither nitrogen oxide nor nitrogen dioxide is nitric acid. Cars have had EGR systems for 50 years now. They produce little to no NOx gases now. Nitrogen dioxide occurs naturally in the atmosphere due to UV exposure from the Sun upon nitrogen. sealover wrote: Making up numbers is not going to help you. sealover wrote: Did they give 'em a good whuppin'? sealover wrote: Nitric acid isn't a fertilizer. sealover wrote: What 'ecosystem'? Plants don't use nitrogen. They can only chemicals like ammonium nitrate, potassium nitrate, or urea to get their nitrogen. sealover wrote: What added nitrogen? From where? sealover wrote: At you least you admit your posts are fake science and filled with buzzwords occasionally. sealover wrote: Buzzword fallacy. sealover wrote: How? Water is not any nitrate. sealover wrote: Nah. The types of plants growing in an area normally change, first as grasses, then shrubs, then trees (assuming there is sufficient water and nutrients for them). sealover wrote: They can't. It's normal. It's not man made. sealover wrote: There isn't that 'kind of thing' is science. Science has no voting bloc. sealover wrote: So the paper was about a meaningless buzzword. Gotit. sealover wrote: Didn't they catch the fella pouring nitric acid into the watershed? sealover wrote: Nitrate isn't a chemical. sealover wrote: Nitrate isn't a chemical. sealover wrote: There aren't any. Nitrate isn't a chemical. sealover wrote: Nitrate isn't a chemical. Carbon isn't organic. sealover wrote: Still can't your head wrapped around reduction reactions either. sealover wrote: Ammonium is not a chemical. sealover wrote: You can't reduce a nitrate. sealover wrote: So why are there so many fish? sealover wrote: Buzzword fallacies. Redundant terms. sealover wrote: Ammonium is not a chemical. Nitrate is not a chemical. You cannot reduce a nitrate. sealover wrote: That you continue to make up buzzwords, cut and paste shit, make up stories about how 'important' you are, and generally try to bullshit your way through life. You are a nothing. sealover wrote: Void argument fallacy. sealover wrote: Tundra is a swamp. That's normal. sealover wrote: Ammonium is not a chemical. Gold mine tailings are generally igneous and sedimentary rock. sealover wrote: Nitrate is not a chemical. sealover wrote: Define The Problem. sealover wrote: Define The Problem. sealover wrote: Nitrate is not a chemical. You can oxidize methane by burning it. Example: gas stove. sealover wrote: Nah. Just like the stove...maybe cook dinner while you have it hot. sealover wrote: Methane is not a 'mess'. It is a naturally occurring gas in the atmosphere. The Parrot Killer Debunked in my sig. - tmiddles Google keeps track of paranoid talk and i'm not on their list. I've been evaluated and certified. - keepit nuclear powered ships do not require nuclear fuel. - Swan While it is true that fossils do not burn it is also true that fossil fuels burn very well - Swan Edited on 02-04-2022 00:31 |
Join the debate Climate Change - Vicious Feedbacks and Worst-Case Scenarios:
Related content
| Threads | Replies | Last post |
| Hey gfm7175 - The Atheist's Worst Nightmare | 32 | 10-04-2024 08:23 |
| Second case of top-secret Biden documents found stored at Staples near the printer | 0 | 12-01-2023 01:46 |
| The Case Of Jesus vs Gautama Buddha Is Giving Some Hint About The Correct Evolution Way | 0 | 22-07-2021 07:31 |
| Another Trump Election Fraud Case Thrown Out | 61 | 09-12-2020 20:12 |
| This is a much bigger issue than a case of mere fraud | 36 | 27-11-2020 23:07 |
