Global warming is caused by ozone depletion, not greenhouse gases
| 27-11-2014 22:27 | |
| peterlward★☆☆☆☆ (69) |
Until you are willing to actually listen to or read the details of what I have very carefully prepared, you have no hope of beginning to understand what I am saying. Thinking of radiation as waves or particles or wave-particle duality is pervasive in physics and has a long rich history that I have researched in great detail. It also has problems. This is all very carefully laid out. When you heat matter, the bonds in matter oscillate as some frequency. The higher the temperature, the higher the frequency and the higher the amplitude. When the body reaches thermal equilibrium, the spectrum of these frequencies is shown by Planck's law. Motion of charge at these frequencies and amplitudes on the surface of matter, induces an electromagnetic field transmitting those frequencies and amplitudes. The frequencies propagate through space without change. The amplitudes radiate and therefore are decreased by 1 over the square of the distance traveled. Everything you see is frequency. Each molecule oscillates at some color. These oscillations travel through air to the rods and cones of your eyes where they cause resonant oscillations of certain cells. These cells send neurologic signals to your brain. This is what Einstein called spooky action at a distance: Oscillations over here cause oscillations over there with no physical thing seen between here and there. This is quantum entanglement. What is traveling in between is frequency. It allows you to see. Your ear works much the same way. This is all explained quite clearly in the video. Reach out. Learn something. Then you can debate from the position of knowledge. |
| 28-11-2014 04:11 | |
| Abraham3 (256) |
peterlward wrote: This is the sort of comment that makes people leery of your opinions. "I'm retired and have a lot of spare time and so I've spent a lot of time studying this and have discovered that one of the most fundamental tenets of quantum mechanics is incorrect. Most scientists will disagree with me but I believe that eventually they'll come around". Perhaps when you say "has problems", you're talking about something known, something benign. Obviously the actual reality of reality (so to speak) has not been definitively described. But if you're trying to say that the workings of quantum mechanics do not require all quantic entities to have a wave-particle duality, I have to step back and refresh my opinion of your technical expertise. peterlward wrote: At some range of frequencies  peterlward wrote: The higher the frequency of the peak of the radiated spectrum. peterlward wrote: Its spectral radiance. peterlward wrote: Their frequency is as susceptible to the Doppler effect as any other EM radiation, but continue. peterlward wrote: No. Within the range of human vision, everything I see is frequency and flux density (power). peterlward wrote: Do radio waves have a color? How about X-rays or gamma rays? peterlward wrote: Only if they fall into the visible spectrum, 400-700 nm. peterlward wrote: Okay, that's far enough. What the F does this have to do with AGW? peterlward wrote: Einstein did use the phrase in reference to quantum entanglement. However, the stimulation of your retina by light is not an instance of quantum entanglement. peterlward wrote: What is traveling in between is a proxy for frequency and amplitude. peterlward wrote: Your ear does not work the same way. Acoustic vibrations are simple compressional waves. Light propagates as oscillating, orthogonal magnetic and electric fields. Edited on 28-11-2014 04:13 |
| 28-11-2014 04:39 | |
| peterlward★☆☆☆☆ (69) |
Yes but it is the oscillations of the acoustic signal that induces oscillation in the cilia of your ear. The frequencies are much lower and the receivers are much larger, being hairs while the receivers in your eye are cells. It is the frequency that is being sensed, not the wavelength. I explain this in the lecture. |
| 28-11-2014 04:56 | |
| peterlward★☆☆☆☆ (69) |
I did not see the upper parts of this comment with my last reply. As for wave-particle duality you have to look at what I have written carefully on the website or talked about in the video. As I show very carefully in the video and on the website, the higher the temperature, the higher the peak frequency of the Planck curve and the higher the amplitude at each frequency. Technically what you see is frequency and each frequency has a brightness. What your eye is sensing is frequency. How much it senses is brightness. Clearly the term color in general usage refers to visible light. But color temperature is used to refer to a much wider spectrum. Frequency applies to all parts of the electromagnetic spectrum. The issue of neurologic signals is how light and what you see gets to your brain. IT is the frequency that sets the primary signal and the brightness that sets how bright the frequency is. You say it is not quantum entanglement. it is exactly what the mathematics of quantum entanglement id trying to understand. You seem to assume that if I disagree with the commonly accepted understandings I am de facto wrong. But you are not willing to look at the details and think about them. Good science is not done by opinion and belief although both have a big role in what a person decides to think about. |
| 28-11-2014 14:58 | |
| Abraham3 (256) |
re your video: "Remarkable how flat temperatures were from 1945 to 1970". Cherry picking. "....explain the inflection points at 1970 and 1998". How about the inflection points at 1910 and 1945? Re your overlay graph of CFC in green on top of temperature in red (Rowland, ozone hole, Montreal). What is the source of your CFC data? Why are scales provided for neither dependent variable? You claim the lag between CFC usage and temperature is caused by the time required for ozone to diffuse from the surface to the stratosphere. Those lags appear to be 5-8 years. Do you have any data supporting the idea that that much time is required? Per the Montreal Protocol, a 100% ban on CFCs was not in place for the non-Article 5(1) nations until the beginning of 1996 and not until 2010 for the Article 5(1) nations. CFC emissions did not stop the instant the Protocols went into effect in 1989. For that matter, the onset of CFC usage was gradual as well. Neither instance supports the sharp turn in temperatures you claim they do. This graphic already displayed temperature - the histogram style data resembling NOAA's graphics. You then overlay what appears to be the NASA GISS temperature profile, presumably to show the peak from Pinatubo. Where are the peaks from Chichon and Agung? Same graphic, ocean heat content vs CO2. Ocean heat content continues to climb after CFC begins decreasing. You claim that is somehow due to CO2 coming out of solution in the ocean. Let me remind you of two points: 1) The temperature of the oceans have risen 0.06C since 1850. That is an increase of absolute temperature (the variable affecting carbonate solubility) of 0.02167%. 2) The increased heat content in the world's oceans represent 90% of the warming the planet has undergone. You seem to be basing your case on the remaining 10% and ignoring the point that that 90% refutes your hypothesis. You conclude this section stating that high but stable levels of ozone are heating the ocean when you have just finished telling us that the stabilization of that level is responsible for the termination of the heating of the planet as a whole. Sorry, but you can't have your cake and eat it too. Large basaltic extrusions and explosive volcanoes also release large amounts of CO and CO2. Why is this not mentioned? Cadle 1980 tells us that chlorine (hydrogen chloride) and bromine appear in volcanic gases in TRACE amounts. The far more common gases include water vapor, CO2, and SO2. http://volcano.oregonstate.edu/book/export/html/156 Consider this a first installment. I really want to see your argument that CO2 warming is physically impossible but I want to see the whole thing. It will take me a while. Edited on 28-11-2014 15:02 |
| 28-11-2014 15:06 | |
| Abraham3 (256) |
peterlward wrote: I'm an engineer, not a grade school student. |
| 28-11-2014 15:20 | |
| Abraham3 (256) |
peterlward wrote: It absolutely is NOT. Wikipedia - Quantum Entanglement Wikipedia - Photoreceptor cells peterlward wrote: You said yourself the odds were high. peterlward wrote: I am working my way through your video now. I have posted comments above about the portion I have watched so far. peterlward wrote: I'm afraid that, watching your video, I am seeing a great deal of what appear to be conclusions drawn on loose, casual and subjective observations. You also appear - with your 1945 cutoff, your failure to mention the CO2 emissions of volcanoes or the triviality of their chlorine and bromine output (particularly that reaching the stratosphere) - to be quite loathe to present information that does not support your argument. I'm sorry, but so far I am not seeing much of a case. |
| 28-11-2014 16:36 | |
| peterlward★☆☆☆☆ (69) |
The warming cycle prior to 1945 can be explained by effusive or weakly eruptive volcanism especially in the early 1930s, the time of the "Dust Bowl" as explained at https://ozonedepletiontheory.info/warm-drought-2012.html. The longer term data do show a cooling around World War 2 that cannot be explained by greenhouse gases. I chose not to complicate the story for the video with these details since the period of relatively constant temperatures from 1945 to 1970 is 25 years, longer than the 16-year period since 1998. But discussion of these details is in the website and in the published paper. The references and scales are all given on the website. The primary reference is Susan Solomon, 1999. In a talk, the most important things to get across are the concepts and it is customary not to litter the graphics with such detail. But it is all there in the published paper and on the website. The apparent lags for sloping lines on a graph of this type are quite dependent on the scales along the y-axis. I chose to have the curves overlap. The references for the lag are on the website. The CFC emission data level off by 1993. These are data, observations. The Montreal Protocol had rapid effects because the most widely used CFCs were made by Dupont and Dupont had a replacement that they were happy to sell. The histogram data are all from NOAA. The black line is ozone depletion data from Arosa Switzerland shown in detail at https://ozonedepletiontheory.info/ozone-depletion.html. There are drops in ozone, increases in depletion, following most eruptions. Pinatubo was the largest eruption since 1912 and the major drop in ozone concentrations after Pinatubo was noted by many authors at the time including Gleason et al., 1993. The continued rise in ocean heat content is caused by the fact that ozone remains depleted compared to pre 1970 levels. We observe that while ozone-depletion is increasing, air temperatures increase, partly due to ground-level industrial ozone pollution. When ozone depletion is no longer increasing but remains high, the air temperatures do not increase but the continued ozone depletion warms the ocean. Ultraviolet radiation penetrates the ocean many meters to tens of meters and is thus its heat is well mixed in. On land, heat absorbed during the day is largely reradiated back into the atmosphere at night. All explained on the website. Now I note that increased ocean heat content, temperature lowers CO2 solubility, providing a potential explanation for the tracking of CO2 and temperature going into and out of ice ages. See next paragraph. The most voluminous gases emitted by volcanoes are water vapor, CO2, and SO2 in that order. Yet following the eruption of Pinatubo, the CO2 concentrations measured at Mauna Loa decreased. The best explanation if that the global cooling of ~0.5oC for three years increased solubility. Sure enough, CO2 at Mauna Loa starts increasing again within 3 years. This is all explained in detail on the website. It is a level of detail that does not fit in a 60 minute talk. Aerosols cool world-wide and the most important temperature change to affect solubility of CO2 is just at the surface, in the mixed layer. Also note the effects of aerosols modeled on ocean cooling in the first two plots on the right side of https://ozonedepletiontheory.info/ice-ages-and-volcanism.html. Pinatubo erupted 3-16 megatons of chlorine compared to 491-921 Mt H20 and 42-234 Mt CO2. One atom of chlorine can destroy 200,000 atoms of ozone because of the catalytic nature of the Chapman Process. Bromine is a similar halogen. References for bromine and ozone depletion are given on https://ozonedepletiontheory.info/ozone-depletion.html. Yes HCl plays a role but because it is water soluble, most authors think it is "rained out" in the eruption cloud. CFCs were developed and used because they do not react with much; they are very stable until ultraviolet light breaks them down in very cold environments such as polar stratospheric clouds. Chlorine, bromine, and fluorine gases erupted by volcanoes are observed to remain in the atmosphere for longer periods than things like HCl. |
| 28-11-2014 16:47 | |
| peterlward★☆☆☆☆ (69) |
I am well aware of the formal definition of quantum entanglement. What I said was "it is exactly what the mathematics of quantum entanglement is trying to understand." How something happening over here can affect something happening over there without any apparent physical connection. How the quantum state of something over here can depend on the quantum state of something over there without any visible connection. And the photoreceptor is reacting to the frequency (energy) of light. The combination of receptors decode color, which is frequency. Thank you for digging deeper. |
| 28-11-2014 17:46 | |
| Abraham3 (256) |
Where do you see a lack of visible connection in the response of photoreceptors and their transmission to the brain? It has absolutely nothing to do with quantum entanglement or the mathematics pertaining thereto. |
| 28-11-2014 18:06 | |
| Abraham3 (256) |
HCL plays a role because it is the primary if not sole route for chlorine to enter the atmosphere via volcanism. It's water solubility IS important because it DOES allow it to be rained out before making it to the stratosphere. Pinatubo put out loads and loads of chorine but virtually NONE of it made it to the stratosphere. The decline in atmospheric CO2 following Pinatubo was not only insignificant (ie, within the noise) but began BEFORE Pinatubo erupted, as may be seen here. Pinatubo erupted 15 June 1991. 
Edited on 28-11-2014 18:08 |
| 28-11-2014 22:56 | |
| Abraham3 (256) |
Let me add that I can believe that the temperature reduction from Pinatubo's aerosols could have increased the ocean's carbonate solubility enough to have caused that very slight dip in levels following the eruption. |
| 28-11-2014 22:59 | |
| Abraham3 (256) |
peterlward wrote: I'm afraid I don't see it. 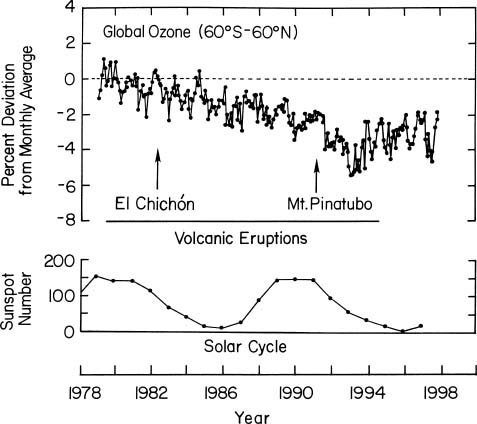 But I'll check Gleason. |
| 28-11-2014 23:05 | |
| Abraham3 (256) |
I find that the Antarctic ozone loss from Pinatubo restricted itself to altitudes below 14 km, well below the stratosphere. http://onlinelibrary.wiley.com/doi/10.1029/96GL02819/abstract |
| 28-11-2014 23:36 | |
| peterlward★☆☆☆☆ (69) |
The lack of physical connection is between the molecule you are looking at and the rods and cones in your eyes. We do not see light in transit. We only see the source. The rods and cones sense frequency. Don't see any disagreement on HCl. As Self et al., 1996) explain "Although 3 Mt of chlorine was erupted (Gerlach and others, this volume) and was potentially available for subsequent participation in ozone-destroying reactions (Turco, 1991), observations by airborne infrared Fourier transform spectrometry of the stratospheric cloud 3 weeks after the June 15 eruption showed little increase in HCl above stratospheric background levels (Mankin and others, 1992; Wallace and Livingston, 1992). Erupted chlorine, as HCl, is highly soluble in water and is very efficiently scavenged by water droplets in the eruption column and rapidly returned to the surface of the Earth as precipitation (Tabazadeh and Turco, 1993). Much of the chlorine may have thus been removed from the atmosphere during or shortly after eruption." Scientists have tracked the growth of the aerosol layer quite precisely. I explain this in some detail at https://ozonedepletiontheory.info/volcanoes-and-climate.html. It took several months to spread around the world and for the particle sizes to grow large enough to reflect/scatter sunlight. The cooling is primarily in 1992 through 1994 and the effect of this cooling is quite clear in the Mauna Loa figure you include and is quite obvious when plotted for the specific time scale. Incidentally, since the aerosol particles must grow in size to be large enough to reflect/disperse sunlight, it is the highest frequencies (shortest wavelengths) that are most affected, i.e. the UV. |
| 28-11-2014 23:48 | |
| peterlward★☆☆☆☆ (69) |
See https://ozonedepletiontheory.info/ozone-depletion.html for the ozone loss and eruptions. This is the data from Arosa, Switzerland, the longest operating ozone recording station and I show this represent mid-latitude ozone depletion quite well (the small blue dots). Volcanic eruptions in the tropics have less effects on Antarctica although Cerro Hudson erupted August 12, 1991, complicating the picture. The tropopause averages 9 km at the poles, well belopw 14 km. |
| 29-11-2014 01:00 | |
| Abraham3 (256) |
The tropopause may start at 9 km at the poles (where it is thinnest and the stratosphere the closest to the surface, but the stratosphere is generally regarded to begin at 30 km.
This has ABSOLUTELY NOTHING to do with quantum entanglement. Period. Your agreement with me on HCL indicates that you agree that volcanic chlorine has little to no effect on stratospheric ozone. Yes? |
| 29-11-2014 01:56 | |
| peterlward★☆☆☆☆ (69) |
"The stratosphere is the second major layer of Earth's atmosphere, just above the troposphere, and below the mesosphere. See the graphs and explanation at https://ozonedepletiontheory.info/very-dynamic-tropopause.html. Einstein called quantum entanglement "spooky action at a distance." That is what happens between the molecule and the inner eye. Quantum entanglement has taken on a rich meaning in the mathematics of quantum mechanics. But the physical process it is describing is "spooky action at a distance." The most direct summary of chlorine after Pinatubo is from Self et al. 1996: "Although 3 Mt of chlorine was erupted (Gerlach and others. this volume) and was potentially avail- able for subsequent participation in ozone-destroying reac- tions (Turco, 1991), observations by airborne infrared Fourier transform spectrometry of the stratospheric cloud 3 weeks after the June 15 eruption showed little increase in HCI above stratospheric background levels (Mankin and others, 1992; Wallace and Livingston, 1992). Erupted chlo- rine. as He!, is highly soluble in water and is very effi- ciently scavenged by water droplets in the eruption column and rapidly returned to the surface of the Earth as precipita- tion (Tabazadeh and Turco. 1993). Much of the chlorine may have thus been removed from the atmosphere during or shortly after eruption." This says much of the chlorine was removed but it does not say how much was left and how much was directly injected into the stratosphere. And from CFCs we know that it takes very little chlorine in the stratosphere to deplete ozone big time. What we do know for sure, was that ozone was depleted following the Pinatubo erupt to the lowest total column ozone value ever recorded by that time. We do not yet understand the whole process. To deplete ozone you in effect interrupt the Chapman process. The options are many. |
| 29-11-2014 05:18 | |
| Abraham3 (256) |
As stated earlier, ozone depletion from Pinatubo chlorine was limited to 14 km and lower. Stratospheric ozone was NOT affected. I am sorry, but you are absolutely wrong about quantum entanglement. Wikipedia - Quantum Entanglement **************************************************************** Quantum entanglement is the function behind the Einstein, Podolsky, Rosen (EPR) paradox. There is no EPR Paradox in faunal visual systems. No step in the optical visualization of our surroundings violates local realism. There is no requirement at any step of the process for correlated quantum states between involved photons. Edited on 29-11-2014 05:49 |
| 29-11-2014 07:08 | |
| peterlward★☆☆☆☆ (69) |
Abraham3 says: The tropopause may start at 9 km at the poles (where it is thinnest and the stratosphere the closest to the surface, but the stratosphere is generally regarded to begin at 30 km. Sorry but the stratosphere is not generally regarded to begin at 30 km. This is quite straight forward. According to Wikipedia: "At moderate latitudes the stratosphere is situated between about 10–13 km (33,000–43,000 ft; 6.2–8.1 mi) and 50 km (160,000 ft; 31 mi) altitude above the surface, while at the poles it starts at about 8 km (26,000 ft; 5.0 mi) altitude, and near the equator it may start at altitudes as high as 18 km (59,000 ft; 11 mi)." Look at https://ozonedepletiontheory.info/ImagePages/tropopause-height.html, some of the best data on tropopause height. |
| 29-11-2014 13:51 | |
| Abraham3 (256) |
You are correct. I was wrong. In my defense, under "stratosphere", Wikipedia has the following:
Emphasis mine, here and below. That would appear to be a typo in their text. I read (or thought I read) "30 kilometers" in that same, first paragraph. My apologies. There is this under Ozone Layer:
though I thought I was referencing a statement that said the stratosphere started at 30 kilometers. Oh well, mea culpa. |
| 29-11-2014 16:11 | |
| peterlward★☆☆☆☆ (69) |
Yes, the top of the stratosphere is close to 50 km. From http://en.wikipedia.org/wiki/Stratopause: "On Earth, the stratopause is 50 to 55 kilometres (31–34 mi) high above the Earth's surface." The tropopause is also very dynamic and has been observed to drop 5 km within 5 hours. See https://ozonedepletiontheory.info/very-dynamic-tropopause.html. The ozone layer, as you know, is not a layer of static gas. It is continually formed and destroyed in the Chapman Cycle driven by UV radiation from the sun. Thus it is most accurately thought of as the region of the atmosphere where the physical, chemical conditions promote the Chapman Cycle. Its height is a function of latitude, being highest in the tropics and lowest in the poles, just like the tropopause. Its height and concentration are also functions of season. See the details at https://ozonedepletiontheory.info/ozone-distribution.html. As I say on the website, I am not convinced that we know the precise chemical process by which volcanic eruptions and even quietly degassing volcanoes deplete ozone. It is clear that chlorine and bromine and possibly fluorine and the rest of the halogens are involved. The process is almost certainly not exactly the same as what we believe happens to CFCs. Furthermore, major increases in ozone concentrations occur before volcanic eruptions and appear to be related to the moment where the magma begins to move (degasses) to the surface. See https://ozonedepletiontheory.info/pre-eruption-ozone.html. As I explain on that page, this does not make sense under current ideas, but the observations appear to be solid as shown in the gif animations on that page. There is so much to learn!! |
| 29-11-2014 17:26 | |
| Abraham3 (256) |
I have not yet had an opportunity to get back to your video. I'm still looking forward to hear your refutation of greenhouse warming from CO2. |
| 29-11-2014 20:20 | |
| Abraham3 (256) |
Back to video: You show a map of world temperature anomalies December 1991 to February 1992. This displays warming over Canada and Scandinavia which you claim is from ozone depletion caused by Pinatubo in the Phillipines. Yet in previous graphics, you claimed that distribution of worldwide distribution of SO2 would take 5-8 years. Angstrom's (Koch's) experiment: Spectoscopy was poor at the time and their observation that the absorption spectra of CO2 and vapor completely overlapped was, as we know now, simply incorrect. Koch's measurement of the absorption difference was incorrect. Angstrom's understanding of the nature of the atmosphere was also faulty. Absorptive saturation at the surface is irrelevant. Radiative heat loss takes place in the uppermost atmosphere. CO2 there - and the expansion of the atmosphere due to heating - both increase trapped infrared and raise the temperature of the atmosphere and the surfaces to which it radiates. I really can't believe you're relying on Angstrom. His work was refuted almost 80 years ago. |
| 29-11-2014 20:28 | |
| Abraham3 (256) |
No one was asking the critical questions? How is matter held together? What is thermal energy? What is temperature in matter? What is temperature in air? What is the relationship between frequency, energy and temperature? How much energy do greenhouse gases actually absorb? How is thermal energy actually transmitted? What is radiation? You've got to be kidding. |
| 29-11-2014 20:48 | |
| Abraham3 (256) |
A more common depiction of the results of Plancks black body radiation spectra. 3 microns wavelength in light is roughly 100 terahertz. So the scale here would read 0 - - 16.7 - - 33.3 - - 50 - - 66.7 - - 83.4 - - 100 tHz (THz?) Edited on 29-11-2014 21:04 |
| 29-11-2014 21:24 | |
| Abraham3 (256) |
The radiation from the Earth does not contain enough frequencies or amplitude to warm the Earth? While sitting there in your living room, put on full long johns, a goose down jacket, a wool cap and some heavy ski gloves. What happens? Your temperature increases from your own radiation. You're claiming that's impossible. A body cannot warm itself? I'm sorry but this contention is simply ridiculous. Edited on 29-11-2014 21:53 |
| 30-11-2014 02:56 | |
| Abraham3 (256) |
Mr Ward, If you want to argue that CO2 is not warming the planet but that it is being warmed by ozone depletion instead, it is not high school students you need to convince, it is the thousands of PhDs worldwide doing real climate science. Among other shortcomings, your arguments lack quantitative substance. "Given that their are only 400 ppm CO2, there are a lot of other gas molecules. And the only way they can get the energy is if it's transferred during the collisions. But in either case, the amount of energy that can cause warming is distributed over so many things that it's just not going to have much effect in actually, physically warming the gas" "A lot of other gas molecules"?!?!? "So many things"?!?!? "Just not going to have much effect"?!?!? Does that sound like science to you Mr Ward? Edited on 30-11-2014 02:58 |
| 30-11-2014 03:16 | |
| Abraham3 (256) |
The absorption of infrared photons by CO2 does not increase its molecular velocity. That's true, for a little less than a millisecond. But molecules that have absorbed photons, emit photons. Half go up and half go down. Eventually those infrared photons will strike the surface where they WILL directly increase sensible heat which can then be transferred back to the atmosphere by conduction/convection and, again, radiation. |
| 30-11-2014 04:10 | |
| peterlward★☆☆☆☆ (69) |
The warming in 1991-1992 is caused by ozone depletion and has nothing to do with SO2 and the aerosols except that the full effect of the aerosols was probably not in force by Jan 1992. The aerosols take months to form and spread, not years. Spectroscopy in the late 1800s was incredibly advanced. The experimental work reported by Samuel Pierpont Langley and many others was thorough and meticulous. Part of the problem today, is that this kind of careful experimental work has not been done for a long time. The Ångström-Koch experiment was straight-forward but not documented in much detail. It is a very sad event for science that this type of straight-forward measurement has not been done more recently. I can predict quite confidently that this experiment would show the same results if done today. While spectral physicists at the time had not detailed the spectral lines of CO2 and H2O that we know today, their results do not depend on this. They simply measured the temperature change or lack of temperature change. And the change was minimal. You then launch into a whole lot of rationalization that has been published by many. Ångström's work has not been refuted. I do not have to rely on Angstroms work but I note that it is the only direct attempt to measure temperature change in the atmosphere due to CO2. As I explain later in the video, the radiation absorbed by CO2 increases the internal energy of the CO2 molecules. Getting from that to increased temperature of a gas is a very inefficient process. Based on this and other physics that I explain, I can predict quite confidently that this experiment done today would show that the temperature of the gas will not be increased much. Do the experiment. Prove me wrong. As for critical questions, these have not been evaluated in detail with regard to climate change and more particularly greenhouse gases, which is the context of this slide. I have been told by some good climatologists that they know of nobody who has looked at these questions in the detail I have. If you know some work and can give me references, I would be happy to modify my statements. Again, the context is greenhouse gases, not general physics or chemistry. As for the depiction of Planck's law, frequency is inversely proportional to wavelength, so you have it backwards. Secondly the scales are normally logarithmic for good reason. I show the frequency and wavelength scales on most of my plots quite intentionally and I have converted most to have frequency increase on the X-axis so that the high-energy is to the right. It is the high energy that gets absorbed in the atmosphere. It is also frequency that we measure, not wavelength. Wavelength is determined mathematically. I show on the website many different plots of Planck's law actually calculated from the law itself for different purposes. It is abundantly clear from plots of Planck's law, that a hotter body radiates more spectral radiance at each frequency and that the frequency of the peak spectral radiance is higher frequency. If you do not see and understand this from the plots, you need to study them more carefully. It is fundamental to thermodynamics that heat flows from hot to cold. Heat does not flow between two bodies at the same temperature (First law of thermodynamics). If you put on lots of sweaters or coats, you will lose heat more slowly and you will feel hotter, but your body temperature of 98.6 will not increase unless new thermal energy is added to the system. A body cannot warm itself. If you do not believe this, you need to study some thermodynamics. The reason most scientists think CO2 warms the air is that the radiation codes used in all models and calculations assume that heat, power, watts per square meter are functions of amplitude and in effect bandwidth and they overestimate the importance of absorption of greenhouse gases. For radiation in space, thermal energy is frequency times the Planck constant. Once you understand this, it is physically impossible for greenhouse gases to have much effect warming Earth compared to ozone depletion. And once you understand this, you find that all observations of global warming begin to make much more sense. I am currently working on a short paper on "The physics of global warming" that explains the physics succinctly. It is quite clear. I am also refreshing my understanding of thermodynamics with an excellent course by Jeff Grossman at MIT (The Great Courses; Thermodynamics: Four laws that move the universe. I highly recommend it. You need to put more energy into understanding the physics than writing scornful posts that are quite uninformed and do not reflect well on you. |
| 30-11-2014 04:16 | |
| peterlward★☆☆☆☆ (69) |
You say "molecules that have absorbed photons, emit photons." That is absolutely untrue although it is widely assumed. Molecules of CO2 in the mid-infrared do not emit energy because electronic transitions do not occur at these energy levels. Furthermore a molecule of CO2 has little thermal mass, is not a black body, and the energy absorbed is a very small amount of the energy contained in the radiation field. |
| 30-11-2014 05:35 | |
| orogenicman★☆☆☆☆ (57) |
The pinatubo eruption did not cause warming anywhere. It actually caused a cooling of the atmosphere over the course of about 1-2 years due to the intruction of sulfur aerosols into the stratosphere. |
| 30-11-2014 06:04 | |
| peterlward★☆☆☆☆ (69) |
Kindly read Robock, A. (2002), Pinatubo eruption: The climatic aftermath, Science, 295(5558), 1242-1244, the paper from which the temperature plot was taken. |
| 30-11-2014 08:24 | |
| orogenicman★☆☆☆☆ (57) |
Did you or did you not already concede: "explosive eruptions also form aerosols (sulfur-based aerosols, to be exact) in the lower stratosphere that reflect and scatter sunlight, causing net global cooling"? |
| 30-11-2014 14:37 | |
| peterlward★☆☆☆☆ (69) |
Look, this kind of very unprofessional mindless dribble is wasting my time and the time of any of the few people who are still following this thread. If you want to have any positive effect on Climate Debate, you need to do due diligence. You need to read, understand, and think carefully about the Science before shooting your mouth off. I have laid out my understanding of the Science very carefully in many different ways and levels of detail, most of which you refuse to read. If your comments are to be of any value to anyone, you need to become informed and to think them through and then explain rationally why you think things are different. I cannot hold your hand at every step of your very reluctant discovery process. You need to have an open mind and to do the work to justify your conclusions. That is how Science works. I go to great length to explain and illustrate the fact that explosive volcanoes deplete ozone causing short-term warming but as the sulfuric-acid aerosols form and grow large enough to reflect and scatter sunlight, they cause a NET cooling. Robock's data documents the warming quite clearly in late 1991 and early 1992 in northern winter. Ozone depletion is greatest during polar winter partly because that is when the total ozone column is greatest. |
| 30-11-2014 18:27 | |
| Abraham3 (256) |
peterlward wrote: But yet you believe that warming in Canada and Scandinavia to be from Pinatubo's chlorine and bromine despite the fact that they were emitted very near to the end of that graphed data, in relatively trace quantities and, at least in the case of the chlorine, very effectively scrubbed by rain. peterlward wrote: Then how did they come to the conclusion that the absorption spectra of CO2 was completely overlain by that of water vapor? peterlward wrote: That says only that he carefully used the tools he possessed. peterlward wrote: The scientific literature is filled with thousands of carefully performed experiments and almost all of them done with more accurate, more reliable, and higher resolution apparatus of all sort than were available to Angstrom, Koch or Langley. peterlward wrote: Than it was not carefully performed. peterlward wrote: I'm quite certain its been done repeatedly in the last few decades, in a thousand high school classrooms. I'd say it doesn't get done much in college laboratories because superior measurements of the absorption spectra have long been available and the conclusion Angstrom drew from his straight-forward measurement - as well as Koch's actual measurements - have since been thoroughly discredited. peterlward wrote: They would not and it would not matter. From http://www.aip.org/history/climate/co2.htm (American Institute of Physics)
peterlward wrote: Do not wonder that your respondents might fall to scorn in the face of such contentions. That Angstrom and those of his era (ie, those with the spectroscopic technology available at the time) believed the absorption spectra of water vapor completely overlay that of CO2 was critical to several faulty conclusions. That CO2's absorption band at 4 microns and most of the wider band at 15 microns (that would be wavelength, the more commonly used unit in discussions of EM radiation) are independent of the presence of water vapor is absolutely critical to understanding the role of CO2 in the greenhouse effect and in our current global warming. Depleted ozone is also known to produce a greenhouse effect and its magnitude has been carefully calculated by many researchers. But it is your claim that CO2 cannot physically produce warming that, to be blunt, is the most outrageous. peterlward wrote: That Koch found a small but non-trivial change in IR absorption in the range of gas concentrations he checked is not challenged. What are challenged are Angstrom's conclusions from the results of that experiment. Angstrom failed to understand the radiative functions of the Earth's atmosphere. As I stated earlier, that 400 ppm of CO2 should make the atmosphere opaque to IR does NOT indicate a saturation of its greenhouse effect. Below 10 km, convection is by far the dominant mode of heat transfer. Above 10 km, radiative effects dominate. The Earth does not radiate to space from its surface but from the 'surface' of its atmosphere where gas density becomes low enough for IR to escape. CO2's absorption spectra is, to a limited extent, widened slightly by both rising and cooling in the Earth's atmosphere. And as the Earth's temperature has risen, the altitude at which density falls sufficient to allow IR to escape, has also risen. This reduces the temperature of the layer from which the Earth's radiation is actually taking place and increases the net radiative imbalance. peterlward wrote: Angstrom made no attempt to measure temperature change in the atmosphere. He measured IR absorption in a glass tube full of CO2. peterlward wrote: But it is a process that takes place and where else does that energy have to go? peterlward wrote: I have no problem (besides accuracy) with Koch's conduct of Angstrom's experiment. My problem - and the problem all your physicist brethren have - is with the conclusions he drew from it. peterlward wrote: The nature of matter in all phases and the functions of thermodynamics have been explored for centuries and are exceedingly well known at all scales. The nature of matter and the physical laws describing and constraining thermodynamics are no different for the elements of the atmosphere than they are any other piece of matter or quanta of energy. The critical questions on this issue are the numerous fine details of the processes affecting our climate, not what is happening to CO2 molecules absorbing photons. I fear you've gotten lost in the rough grass. peterlward wrote: What makes you think such fundamentals would differ in a climate context? peterlward wrote: I am an ocean engineer and my specialty is acoustics. I am well aware how to deal with frequencies. Wavelength (or wave number) is a far more common unit in discussions of EM for the same reason astronomers rarely speak of distance in miles. Ten microns is a great deal handier a term than is Thirty trillion hertz and a great many more actions are physically dependent on wavelength (actually, all of them) than are on frequency (none of them), an artificial term (s^-1 means nothing away from a human clock). peterlward wrote: All your choice. peterlward wrote: I think they're a BOGO (buy one, get one free) peterlward wrote: Wavelength is a physical parameter. It's numeric value may change with different units but the physical reality - the length of a single wave - does not. Frequency, on the other hand, includes the completely arbitrary second (or minute or hour or week or month or year or century or milennia) and the actual value of an oscillation's frequency DOES change depending on the time base used. Wavelength is the more fundamental parameter and it is the physical characteristic that controls all oscillating signal interactions. peterlward wrote: My example was not analogous but I was feeling a bit peremptory since neither was yours. Let's take another chestnut. I have a water tank which has a fill line and a drain line. I open both to some extent and leave them there. The tank fills with water till hydrostatic pressure causes enough flow in the drain line to match the incoming fill. The tank has reached equilibrium. What happens if I close the drain valve slightly? The water level rises to a new equilibrium. The same is true of the Earth. The Earth is not warming itself. It is moving towards a higher thermal equilibrium with the sun in response to the increased retention of IR. peterlward wrote: Of course it does. It is only net flow that is zero. peterlward wrote: Which is precisely what your metabolism is doing. peterlward wrote: I aced two semesters of thermodynamics and another of non-equilibrium heat transfer. The Earth is not a body warming itself. peterlward wrote: The reason they think CO2 can warm air is this:  And WHAT, pray tell, do you mean when you say they "overestimated the importance of absorption of greenhouse gases"? You're aware, I assume, of the difference between the Earth's blackbody temperature and actuality. To what can you attribute that other than the greenhouse effect? peterlward wrote: I hope you found that very tempting because you skipped a great deal of fundamental physics to get there. Planck's constant of proportionality: epsilon=h\nu, applies to "an oscillator". A gas, whatever the temperature, is not an oscillator. EM energy behaves quite differently in the presence and absence of matter. In the latter, it will follow the non-linear Euler–Heisenberg Lagrangian equations while in the latter, the linear equations of Maxwell to which Planck was restricted. peterlward wrote: Good. Ask him what he thinks of the idea that photons cannot warm gasses. peterlward wrote: Don't we all. Edited on 30-11-2014 19:17 |
| 30-11-2014 18:56 | |
| Abraham3 (256) |
peterlward wrote: Molecules of all sort routinely absorb and emit photons. The more they have absorbed, the higher their energy level. The higher their energy level, the more likely they will emit one or more photons. I am aware of quantum restrictions on those emissions, but whatever do you mean by "Molecules of CO2 in the mid-infrared"? Did you mean to say that CO2 will not emit a photon outside its emissions bands? Were you under the impression that the world's climate scientists were not aware of the molecular weight of CO2, it's absorption and emission spectra or what 400 ppm actually means? You will not convince the world's climate scientists with such arguments. |
| 30-11-2014 19:31 | |
| orogenicman★☆☆☆☆ (57) |
peterlward wrote: Excuse me? You did not make clear for whom your comments were meant, but I asked a simple question for a very simple reason. I would have thought that someone with your credentials would have thicker skin than this. I don't have a PhD, though I am a geologist who specializes in groundwater hydrogeology , have published in the Journal of paleontology, have written hundreds of technical reports for 13 states and for industry, and have 25 years under my belt - so I too have paid my dues. I do think carefully, and trust me, you do NOT need to hold my hand. Now that we have the hand waving out of the way, can we move on? Your hypothesis does not explain how short term warming due to volcanic aerosols, which is followed by short term cooling, affects long term global warming we see in the temperature records. I understand that long term eruptions such as the Siberian trapps released massive volumes of not only SO2, but CO2 as well over a relatively long period of geologic time, and appear to have had a major effect on global temperatures. But as we have seen, and as you have pointed out, recent (i.e., the last few thousand years, at least) eruptions have had but minimal impact on global temperatures. Yes, we had the year without summer, thought to have been caused by the eruption of Mt. Tambora. But global temperatures rebounded within a year or two. So where was the warming in that event? Where was the warming that followed the eruption of Mt. Pinatubo. Every temperature graph I have seen doesn't show any immediate warming that you describe above. It shows a downward spike in temperatures followed by a rebound, indicating that the influence of that eruption was brief. Lastly, you mention in your paper that volcanic eruptions that occurred at the end of the last ice age initiated that end. I am not a volcanologist, though I have held an interest in the subject all of my life, so I have to ask to what eruptions are you referring? I have never seen a list of eruptions from 13,500 years ago that were large enough or lasted long enough to have produced enough ghgs to have ended the ice age. Can you provide us with such a list? On average, in your opinion, how long do SO2 aerosols reside in the atmosphere? If they have such an appreciable impact on ozone (and I don't doubt that some impact exists), allowing so much more UVB to reach the Earth's surface, why don't we see past events causing genetic mutations in large numbers of species? Why are there no extinctions associated with such events? Where the evidence of such impacts in the geologic record? Now, surely you must understand that as the promoter of this hypothesis, though you apparently consider these questions to be trival, drivel, or beneath your station in life, they are nonetheless valid questions which you need to address if you expect to gather support for it. And my advice to you is to not take these questions personally. They are fairly mild, in my opinion, compared to what others can and will no doubt ask. Edited on 30-11-2014 19:34 |
| 30-11-2014 19:49 | |
| peterlward★☆☆☆☆ (69) |
You do not listen and you certainly do not learn. Again: The warming was caused by the observed ozone depletion. The heat quantities involved add up. Volcanic eruptions are clearly observed to cause ozone depletion. The precise cause of this observed depletion has not yet been adequately defined in my opinion but chlorine and bromine play major roles according to the literature. When you directly measure the change in temperature of a gas caused by absorption of infrared energy, you do not need to make any assumptions about what in the gas is absorbing or whether the absorption spectra of different gases overlap. You either see an increase in temperature or you do not. This applies also to the quote you give from Spencer Weart and the paper he wrote with Raymond Pierrehumbert. Their arguments are a lot of hand waving that obscure the issue. The carefully performed experiments in the scientific literature over the past century focus primarily on the spectral lines. As I said these have been tabulated with great precision. They are used heavily for spectroscopy of distant galaxies and close at hand. They do not focus on the issue of increased temperature due to absorption. I called Spencer Weart and asked him if he knew of any such studies aimed at the temperature increase. He did not. The thermodynamics of solids, gases, and radiation are quite different in detail although they are interrelated. Assumptions about radiation of heat by gases at atmospheric temperatures are not well established. I have already explained this. Gases do not radiate in the infrared bands involved with greenhouse gases. Gases radiate through electronic transitions and these do not occur at the frequencies (wavelengths) involved with greenhouse gases. My PhD was first and foremost in seismology and I understand seismic and acoustic waves in rich detail. But waves are a property of solid matter and compressed fluids and they cannot occur in space. This is explained in the video and in the text. Can you prove otherwise? Many scientists tried for many decades and have not been successful. We have lots of mathematics that describes waves in space, but we do not observe waves in space. You are going in circles and not listening to anything I have said. Energy in radiation is equal to frequency times the Planck constant. You can see this is a reality yourself as I describe in the video. This takes no assumptions about photons or any physics. We all know that nuclear radiation is much more energetic, can damage us more, than radio transmissions. Energy causes change and the changes observed at various frequencies of radiation all fit this relationship. If you aced thermodynamics then why did you not correct my quote of the first law. It should have been the zeroth law. And according to Grossman: "The zeroth law of thermodynamics states that if objects A and B are each in thermal equilibrium with respect to object C, then A and B are in thermal equilibrium with each other. From the zeroth law one can define temperature as that which is equal when heat ceases to flow between systems in thermal contact." |
| 30-11-2014 20:27 | |
| orogenicman★☆☆☆☆ (57) |
peterlward wrote: While it is true that acoustic waves do not propagate in the vacuum of space, it is not true that light waves (i.e., IR) do not propagate in space. Most certainly they do, otherwise we would not be able to OBSERVE the stars, galaxies, nor even the sun, nor feel the sun's thermal energy (i.e., heat). The above claim warrants a facepalm, Peter. Perhaps you should rethink the above. |
Join the debate Global warming is caused by ozone depletion, not greenhouse gases:
Related content
| Threads | Replies | Last post |
| The "radiative Greenhouse effect" does not exist | 140 | 15-04-2024 19:43 |
| 'Greenhouse' Effect? | 49 | 30-11-2023 06:45 |
| The SCIENCE of the "Greenhouse Effect" | 291 | 05-11-2023 22:46 |
| How covid 19 vaccine caused Jamie Foxx to become blind and paralyzed | 2 | 08-06-2023 03:23 |
| Nitrate Reduction - Powerful Greenhouse Gas Emission AND Alkalinity | 102 | 05-06-2023 13:19 |